Folia Microbiol. 32; 345- 348 ( 1987)
J. FLEGRa, J. CERKASOVa, J: KULDAa, J. TACHEZYa and J. ŠTOKROVÁb
a Faculty of Science, Charles University, 128 44 Prague 2 b Institute of Molecular (enetics, Czechoslovak Academy of Sciences,
166 37 Prague 6
Received July 31,1986
ABSTRACT. Twelve rnetronidazole-resistant and twelve metronidazole-susceptible strains of Trichomonas vaginalis were tested for the presence of dsRNA. Three resistant and five susceptible strains were found to contain dsRNA which indicated that metronidazole resistance does not correlate with the absence of dsRNA. Electron microscopy showed the homogenates of all dsRNA-positive strains to contain virus-like particles 32-38 nm in diameter, while no such particles were found in the dsRNA-negative strains. A mutual relationship between the dsRNA and virus-like particles seems to exist.
Some strains of Trichomonae vaginalis, a protozoan parasite living in the human urogenital tract, contain double-stranded RNA (dsRNA), often in amounts comparable to that of nuclear DNA (Wang and Wang 1985, Flegr et al. 1985).
The biological significance of dsRNA in trichomonads is not known. Its presence was shown not to be connected with virulence (Flegr et al. 198). The results of Wang and Wang (1985), however, suggest a possible correlation between absence of dsRNA and resistance to metronidazole, a drug most often used in human trichomoniasis. Only two of the total of 35 strains tested did not contain dsRNA and they were the only metronidazole-resistant strains present in the sample. These results, together with the finding of a nuclear DNA sequence homologous with the dsRNA lead the authors conclude that dsRNA might be a physiological constituent of the T. vaginalis cell.
In contrast, Flegr et al. (1986) suggested a viral origin of dsRNA in T. vaginalis on the basis of the following observations.
l. dsRNA is found in some strains of T. vaginalis only. 2. The isolated nucleic acids contain several distinct populations of dsRNA molecules of various sizes, which resembles the situation in the segmented genome of dsRNA viruses. 3. The behaviour of dsRNA during fractionation of the homogenate using differential centrifugation suggests that it is particle-bound.
In this paper we report the presence of virus-like particles in dsRNA- positive strains of T. vaginalis` and the absence of any correlation between dsRNA and metronidazole resistance.
* After this paper had been accepted for publication the occurrence of virus-like particles in dsRNA-positive trichomonads was reported by others (Wang A.L., Wang C.C.: The doubla stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc.Nat.Acad. Sci. USA 83, 7956- 7961, 1986).
MATERIALS AND METHODS
Organisms. The strains employed are listed in Table I. All metronidazole resistant strains were isolated from patients who were not cured by a standard treatment with metronidazole or tner 5-nitroimidazole derivatives. The susceptible strains were isolated from patients cured by single standard metronidazole treatment (Entizol, Polfa, 20 mg three times a day for a 7 days). All strains were axenized- and were maintained as cryostabilates ( % dimethyl sulfoxide, liquid nitrogen). Their susceptibility to metronidazole
Table I. Strains used
Strains Origin Metronidazole Presence
MLC,,g/mLs a of dsRNA
Resistant to metronidazole
IR-78 Vienna, Austria (Meingassner and Thurner 1979) 100 -
BO Gothenburb, Sweden (Forsgren and Forssman 1979) 50 -
Boston Boston, USA (Muller et al.1980) 25 -
Albany Albany, USA (Muller et al.1980) 25 -
Fall River Fall River, USA (Muller et al.1980) 100 +
MRP-2MT Prague, ČSSR (Kulda et al.1982) 100 -
SA USA (Meingassner 1983) 79 +
141 ditto 50 -
316 ditto 100 -
NAD Bratislava, ČSSR (Valent et al.1985a) - +
K Bratislava, ČSSR (Valent et al.1985b) 70b -
HL-2MT Liberec, SR (Kulda, 400 unpublished results) 400 +
S'encitive to metronidazole
A1 Vienna, Austria (Meingassner and Tllurner 1979) 3.5 +
TV 5-27 Prague, ČSSR (Kulda et al.1982) 2.5 -
TV 7-37 ditto 20 -
TV 10-02 ditto 3.1 +
TV 14-85 ditto 9.9 -
TV 17-48 ditto 12.5 -
TV 67-77 ditto 12.5 -
TV 73-87 ditto 3.9 +
TV 79-49 ditto - +
TV 85-08 Prague, ČSSR (Kulda, unpublished results) 6.3 +
TV 87-64 Prague, ČSSR (Kulda et al.1982) 5.0 -
TV 99-51 ditto 12.5 -
a Mean of 3 independent estimations.
b Measured by P. Demeš (Bratislava), personal communication.
was determined by measuring the minimal lethal concentration (MLC) in vitro (Meiagassner .and Thurner 1979).
Cultivation. The strains were isolated according to Kulda et al. ( 1982) and grown in the trypticase, yeast extract, maltose (TYM) medium (pH 6.0) without antibiotics, containing inactivated horse serum (10%) (Diamond 1957). The cells :to be homogenized were cultivated in the medium without agar.
Susceptibility to metronidazole. The susceptibility was tested on microtitration plates according to Meingassner and Thurner (1979) in TYM medium without agar containing 10 % pre-colostrum calf serum (Bioveta, Ivanovice na Hane, Czechoslovakia). MLC was determined on the basis of loss of cell motility after a 48-h exposure to metronidazole under aerobic conditions.
Nucleic acid isolation. About 10E8 late-exponential-phase cells were washed with 0.8 % NaCl and extracted with phenol (pH 4.0) (Zasloff et al. 1978). The isolated nucleic acids contained only RNA (Plate 1 ). The precipitted nucleic acids were washed with ethanol and dissolved in 40 ,L of TE buffer ( 10 mM Tris-HCl, 1 mM EDTA, pH 8.0).
Nucleic acid electrophoresis. The nucleic acids were separated in 1 % agarose using Tris-borate buffer (Maniatis et al. 1983); dsRNA of a type 3 human reovirus (strain Dearing) (Ramig et al. 1977) was used as standard.
Electron microscopy. Cell homogenates prepared by disintegration of T. vaginalis in a Potter-Elvehjem homogenizer were stored at -20 oC and negatively stained with phosphotungstic acid (Frank et al. 1978).
RESULTS
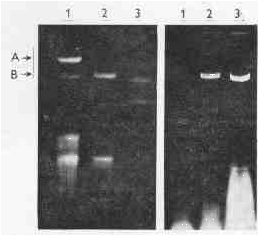
Twelve metronidazole-susceptible and twelve metronidazole-resistant strains were tested, of which only eight (five susceptible and three resistant) contained dsRNA. The mobility of dsRNA in agarose was the same in all positive strains (Plate l ).
PLATE 1. Electrophoresis of nucleic acids of T. vaginalis; left: 1 - deproteinized with phenol at pH 8.1, 2 - deproteinized with phenol at pH 4.0, 3 - a standard of the type-3 reovirus dsRNA (strain Dearing), molar mass of the longest reoviral segment was 2.5 Mg per mol; right: 1 - strain BO, 2 -strain Fall River, 3 - strain TV 10-02 (strains BO and Fall River are metronidazole-resistant, TV 10-02 is metronidazole-sensitive), isolated with phenol at pH 4.0; A - DNA, B - dsRNA.
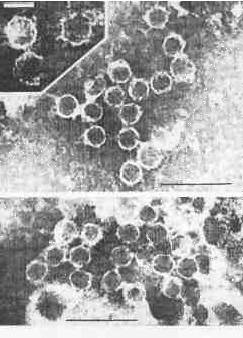
Electron micrographs of cell homogenates of dsRNA positive strains showed numerous hexagonal particles (32-38 nm in diameter, uniform in size and shape (Plate 2). These structures resemble icosahedral virus-like particles. They were repeatedly found in all five dsRNA-positive strains but in none of the five dsRNA-negative strains tested.
PLATE 2. Virus-like particles in cell homogenates of T. vaginalis stains containing dsRNA (negative staining by phosphotungstic acid); black bars represent 100 nm, white bar 30 nm.
DISCUSSION .
In this paper we demonstrate the existence of dsRNA in a number of clinical isolates of T. vaginalis, confirming earlier reports (Wang and Wang 1985; Flegr et al. 198) and the presence of virus-like particles in the homogenates of all dsRNA-containing isolates tested. This finding indicates that the dsRNA detected in this organism is of viral origin. The size (and shape) of the virus-like particles is similar to that of some dsRNA mycovirus capsids (Ushiyama 1985)., Virus-like particles have been detected in a number of parasitic protozoa (Schuster and Durnebache 1971 ; Mattern et al. 1972) but their significance remains unknown.
Our results also confirm earlier reports which demonstrated that dsRNA
is not present in all isolates (Flegr et al.1986; Wang and Wang 198) and show that virus-like particles are absent in organisms without dsRNA.
Our study included clinical isolates with various levels of metronidazole susceptibility. No correlation between this property and the presence of dsRNA or of virus-like particles was found, however, in contrast to a report in which the absence of dsRNA was found to be correlated with metronidazole resistance (Wang and Wang 198). A possible explanation for this discrepancy might reside in the selection of susceptible isolates by the latter authors which originated almost exclusively (31 of 33 isolates) from a single source (National Defense Center, Taipei, Taiwan) and thus may not represent independent strains. This could also explain the different frequency of the dsRNA positive strains in the two samples (33 and 94 %, respectively).
The authors express their thanks to Dr. J.G. Meingassner (Sandoz Research Institute, Austria) for the strains of T. vaginalis isolated in Austria, Sweden and the USA, and to Dr. P. Demeš (Institute of Parasitology Comenius University, Bratislava) for providing the strains isolated in Bratislava. The generous gift of reoviral dsRNA from Dr. M. Rosembergová (Institute of Virology S'lovalc Academy of Sciences, Bratislava) is also gratefully acknowledged.
REFERENCES
Diamond L.S.: The establishment of various trichomonads of animals and man in axenic culture. J.Parasitol. 3, 488-490 (1957).
FLEGR J., ČERKASOV J., KULDA J., ŠTOKROVÁ J.: Occurrence of double stranded RNA in Trichomonas vaginalis, p. 43 in Abstr. Internat.Symp.Trichomonads and Trichomoniasis, Prague 1985.
FLEGR J., ČERKASOV J., ČERKASOVOVÁ A., KULDA J., ŠTOKROVÁ J.: Double stranded RNA in
Trichomonas vaginalis, pp. in Trichomonads and Trichomoniasis, Part 1 (J. Kulda, J. Čerkasov, eds.). Acta Universitatis Carolinae Biologica, in press 1987.
FORSGREN A., FORSMANN L.: Metronidazole resistant Trichomonas vaginalis. Brit.J.Vener.Dis. , 351- 353 ( 1979).
FRANK H., SCHWARZ H., GRAF T., SCHAFER W.: Properties of mouse leukemia viruses. XV. Electron microsopic studies on the organization of friend leukemia virus and other mammalian C-type viruses. Z.Naturforsch. 33c, 124-138 (1978).
KULDA J., VOJTCHOVSKÁ M., TACHEZY J., DEMEŠ P., KUNCOVA E.: Metronidazole resistance of Trichomonas vczginalis as a cause of treatment failure in trichomoniasis. Brit.J.Vener.Dis. 58 , 394-399 (1982).
MANIATIS T., FRITSCH E. F., SAMBROOK J.: A Laboratory Manual, Molecular Cloning. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 1983.
MATTERN C.F. DIAMOND L.S., DANIEL W.A.: Viruses of Entamoeba histolytica. II. Morphogenesis of the polyhedral particle (ABRM2-HK-9) -> HB-301 and filamentous agent (ABRM)2 -> HK-9. J.Virol. 9, 342-358 (1972).
MEINGASSNER, J.G., THURNER J.: Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazols. Antimicrob.Agents Chemother. 15, 254-257 (1979).
MEINGASSNER J.G.: Nitoimidazole resistance in trichomonads. Wiad.Paraztol. 29, 151-154 (1983).
MULLER M., MEINGASSNER J., MLULLER W.A., LEDGER W.J.: Three metronidazole-resistant
strains of Trichomonas vaginalis from the United States. Am.J.Obstet.Gynecol. 138, 808-812 ( 1980).
RAMIG R.F., CROSS R.K., FIELDS B.N.: Genome RNAs and polypeptides of reovirus sorotypes 1, 2, and 3. J. Virol. 23, 726-733 (1977).
SCHUSTER F.L., DURNEBACHE T.H.: Formation of bodies associated with virus-like particles in the amoeboflagellate Naegleria gruberi. J. Ultrastructure Res. 36, 659-668 (1971).
USHIYAMA R.: Viruses in fungi and eukaryotic algae: their possible origin and evolution. Microbiol..Sciences 2, 181-184 ( 1985).
VALENT M., DEMEŠ P.,ŠTĚTKOVÁ D., BALOGHOVÁ E.: Two clinical cases of urogenital trichomoniasis resistant to nitroimidazole derivatives. (In Slovak) Českosl.Gynecol. 50, 696-697 (1985a).
VALENT M., DEMEŠ P.,ŠTĚTKOVÁ D.: Unsuccessful treatment of a patient with urogenital trichomoniases, caused by resistance of the agent to nitroimidazole derivatives. (In Slovak) Bratislavské Lékar.Listy 83; 156-161 (1985b).
WANG A.L., WANG C.C.: A linear double stranded RNA in Trichomonas vaginalis. J.Biol.Chem. 260, 3697-3702 (1985).
ZASLOFF M., GINDER. G.D., FELSFELD G.: A new method for the purification and identification of covalently circular DNA molecules. Nucl.Acids Res. 5, 1139-1152 (1978).