Nitrated polycyclic aromatic hydrocarbons
1. Introduction
Nitrated polycyclic aromatic hydrocarbons (NPAH) belong among highly dangerous mutagenic compounds, which are associated with the increasing occurence of cancer diseases.The interest in these substances have grown after 1978, when it was discovered that NPAH could be formed in polluted air by reactions of polyaromatics with nitrogen oxides. Some of NPAH are directly emmited to the atmosphere by diesel and petrol engines. Nitrated polycyclic aromatic hydrocarbons are responsible for a high proportion of the "direct-acting" mutagenicity of air and diesel particulate extracts, when tested in Amesí bioassay. Great effort have been made to detect NPAH in different kinds of environmental samples. Attention was also being paid to the analysis of another highly mutagenic derivatives, which can be formed during metabolic transformations.
2. Origin and occurance of nitrated polyaromatics
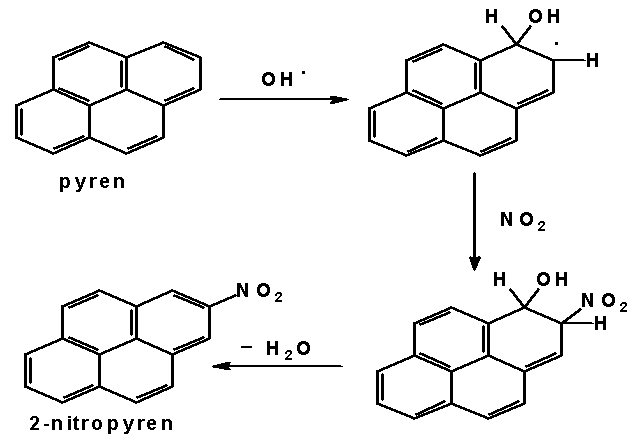
Nitrated polycyclic aromatic hydrocarbons have been found in air, diesel and petrol engine exhausts, in carbon black, exhaust from waste incineration plants, in sediments, cigarete smoke and some foodstuffs. Diesel engine exhaust belongs to the most important sources of NPAH in the air. More than 50 different nitrated polyaromatics have been identified in diesel exhaust samples. The most abundant substance is 1-nitropyrene, although we can find 3-nitrofluoranthene, 8-nitrofluoranthene, anthracene and phenanthrene nitroderivatives in these samples as well. Further investigations have been focused on the study of mechanism of NPAH atmospheric formation. Typical representatives of NPAH formed exclussively by atmospheric reactions are 2-nitropyrene and 2-nitrofluoranthene. Two different mechanisms of the formation of these substances are assumed. The first one assumes iniciation of the reaction by an attact of OH radical on gaseous hydrocarbon, followed by addition of nitrogen dioxide and termination by elimination of water molecule. This mechanism, occuring mainly in the daytime, is shown below. The second mechanism occures in the night-time when reaction between dinitrogen pentaoxide and hydrocarbon is assumed.
Mechanism of 2-nitropyrene formation in the atmosphere

3. Biological activities of nitrated polyaromatics
When analyzed in Ames assays, many of NPAH have been found to be mutagenic even in the absence of exogenous metabolic activation. Some of them, such as dinitropyrenes, are among the most potent mutagens ever tested. Biological activity of NPAH was a topic of more than 100 articles, mutagenic and carcinogenic properties of selected NPAH are summarized in the table below. NPAH can enter the body by inhalation, absorption through the skin and through intestinal tract. They are probably reduced by nitro-reductases in the liver to form methaemoglobin - inducing substances, e.g. nitroso derivatives and N-hydroxylamines. However, these intermediates are further reduced to the corresponding amino aromatic compounds, which are excreted in urine in free form or after acetylation. The cellular reduction of NPAH is catalysed by NADPH-cytochrome P450 reductases. In addition, C-hydroxylation of the aromatic ring system can occure and further methabolic derivatives of the NPAH can be formed by N-N dimerization of the reduced intermediates. The toxic effects of various nitroaromatic compounds are attributed to the formation of the above mentioned free radicals as a result of enzyme activity. Their most important reactions are with the cellular macromolecules, especially with proteins and nucleic acids. These reactions may explain the mutagenic and carcinogenic effects of NPAH independently on the effects of the corresponding APAH. Genotoxic properties of NPAH generally depend on the structure of parent PAH and on the number and position of the nitrogroups. The metabolic activation has been shown to involve nitroreduction, nitroaromatic ring oxidation, N-hydroxylamine O-acetylation, or, in some cases a combination of all three pathways.
| Substance | Mutagenicity | Carcinogenicity |
|---|---|---|
|
1-nitropyrene |
++ |
-/+ |
|
2-nitropyrene |
++ |
-/+ |
|
4-nitropyrene |
++ |
+ |
|
1,3-dinitropyrene |
+++ |
+ |
|
1,6-dinitropyrene |
+++ |
+ |
|
1,8-dinitropyrene |
+++ |
+ |
|
1-nitronaphtalene |
-/+ |
- |
|
2-nitronaphtalene |
-/+ |
+ |
|
9-nitroanthracene |
-/+ |
? |
|
2-nitrofluorene |
+ |
+ |
|
3-nitrofluoranthene |
+++ |
+ |
|
6-nitrobenzo[a]pyrene |
++ |
-/+ |
|
7-nitrobenzo[a]anthracene |
-/+ |
-/+ |
|
6-nitrochrysene |
+ |
+++ |
- inactive; -/+ very weak; + weak; ++ moderate; +++ strong
4. Methods of determination of nitrated polyaromatics
4.1 Sampling
The particle-bound NPAH in the air can be collected on glass-fibre filters and subsequently desorbed with organic solvents. Extraction is usually done in Soxhlet aparature using dichloromethane, in some cases by sonication. When high-volume sampling is used, some incorrect results can be obtained because of NPAH formation during sampling. Some types of denuders can also be used for sampling. The blood sample is withdrawn from the arm vein, plasma is immediately separated by centrifugation and ethanol is added to cause deproteinization. NPAH presented in a free forms are extracted using 2,2,4-trimethylpentane (iso-octane).
4.2 Preliminary separation and preconcentration
Because of complexity of environmental, biological or other samples, analytical methods for the determination of NPAH often incorporate a preliminary fractionation. Usually it is done using high-presure or open bed column liquid chromatography in a normal phase systems. Solid-phase extraction or supercritical fluid extraction are also suitable methods for extraction and preconcentration.
4.3 Chromatographic methods
Chromatographic methods are the most frequently used techniques in the analysis of NPAH both in environmental and biological samples, because of their selectivity, sensitivity and a high sample throughput. Detection and characterization of seven NPAH by fluorescence quenching after TLC separation has been described already in 1978. In HPLC techniques the reversed-systems with C18 stationary and methanol/water or acetonitrile/water mobile phases are usually employed. The commonest detector used is a spectrophotometric detector in UV region, the sensitivity of this detection for NPAH is very low. It is possible to achieve higher sensitivity using electrochemical detection. The most sensitive detection techniques in HPLC are probably techniques based on fluorescence or chemiluminiscence. The common disadvantage of these methods is the need of reduction of NPAH to the corresponding amino derivatives. Reduction can be performed "off-line", outside the HPLC system or better "on-line" in HPLC system. Multidimensional HPLC offers interesting possibilities in analysis of NPAH. The most powerful tool for the identification and determination of NPAH is gas chromatography with mass detection (GC-MS) which can analyze more than 50 compounds in a sigle run. The other detectors used in GC analysis of NPAH are TEA (thermal energy analyzer), NPD, ECD a FID.
4.4 Polarographic and voltammetric methods
Because of easy polarographic reducibility of nitro group , trace amounts of NPAH can be determined using modern polarographic and voltammetric techniques, namely differential pulse polarography on dropping mercury electrode or differential pulse voltammetry on hanging mercury drop electrode. A relationship between polarographic half wave potential and mutagenicity of NPAH is very interesting. The knowledge of the mechanism of the polarographic reduction can give us a useful clue in elucidation of the mechanism of their metabolic transformation.
4.5 Spectrometric methods
NPAH can be determined using spectrophotometry in UV region. Fluorimetry, which is a useful tool in the detection and quantification of PAH cannot be applied directly to NPAH, which are almost non-fluorescent. However, some NPAH are known to phosphorescence strongly at low temperatures.