L. D. Quin, Xiao-Ping Wu, N. D. Sadanani,
I. Lukeš, A. S. Ionkin, R. O. Day:
"Synthesis, Fragmentation, and Photorearrangment of Neopentyl and Adamantyl Phosphonates
in the 2,3-Oxaphosphabicyclo[2.2.2]Octene System."
J. Org. Chem. 59, (1994) 120-9
► DOI: 10.1021/jo00080a020,
► PDF
Abstract:
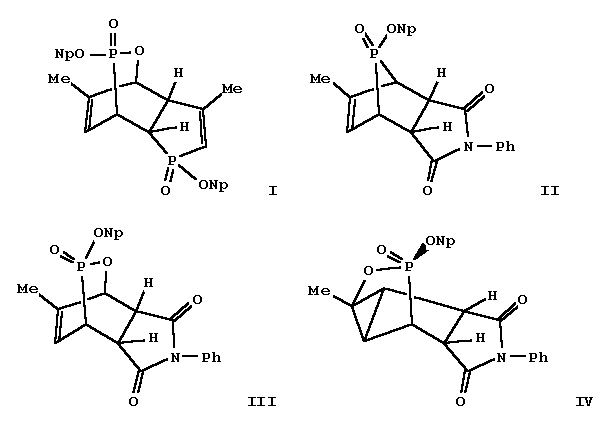
Precursors for the generation of neopentyl and 1-adamantyl metaphosphates
were prepd. by the insertion of oxygen into a ring carbon-phosphorus bond
of some 7-phosphanorbornene derivs.
The stereochem. of the resulting products, which possess the
2,3-oxaphosphabicyclo[2.2.2]octene ring system,
was established by NMR spectroscopy, and by X-ray anal.
in I (Np = neopentyl).
The O-insertion (by MCPBA) generally proceeds
with retention of phosphorus configuration, but in one precursor
with a syn-neopentoxy group a minor product from an inversion process
was isolated. The 7-phosphanorbornene isomer
II with the uncommon anti neopentoxy structure was synthesized
by rearrangement of the syn isomer; O-insertion gave
exclusively compd. III, the product of retention,
identical to the minor product from the syn isomer.
Conditions were developed for the photochem. fragmentation of the precursors
at room temp. to release the metaphosphates
[ROP(:O)2]
(R = neopentyl, 1-adamantyl); these highly
reactive species were trapped as phosphates when alcs. were included
in the medium. Thermal fragmentation also was effective for generating
the neopentyl ester. Irradn. was also performed at -75°C
in an attempt to stabilize the metaphosphates so as to allow their spectral
characterization, but a rearrangement occurred to give a novel tricyclic compd.,
e.g., IV from photorearrangement of III.
In this rearrangement, the ring oxygen shifted stereospecifically
to the adjacent sp2 carbon, and a cyclopropane ring was formed.