
The main topic of our research is a coordination chemistry of transition metal and lanthanide ions in aqueous
solution, synthesis and study of new ligands and their complexes suitable for biomedical applications.
In the clinical medicine, a lot of pharmaceuticals based on metal-containing compounds are daily used.
Transition metal ions are often highly toxic. Beside it, they are deposited in tissues.
For applications in the clinical use, they mostly cannot be used in form of simple salts and toxic metal ions must be bound in a
thermodynamically and kinetically stable complexes. The thermodynamic aspect guarantees a stability of the complex
in the presence of concurrent biogenic ligands and ions. The kinetic inertness further decreases probability of
the complex decomposition. Basic research on this field is aimed on design of new ligands forming metal complexes
showing sufficient in vivo stability. The complexes must hold their expected function in a treatment and a diagnosis.
This group of complexes is mainly applied in radiomedical applications (radiodiagnostics and radiotherapy),
in magnetic resonance imaging (MRI) and in other imaging methods. All these methods together are called
Molecular Imaging.
Molecular Imaging
Molecular imaging represents a group of imaging techniques used in the medicine and the molecular biology (in the medicine often called CT – Computer Tomography). To reach better contrast of the image, contrast agents are often applied. Members of the family of Molecular Imaging methods are MRI (Magnetic Resonance Imaging), radiodiagnostic methods (SPECT – Single-Photon Emission Computer Tomography, PET – Positron Emission Tomography), optical imaging methods (luminescent and fluorescent markers) and an ultrasound.
Magnetic Resonance Imaging
The Magnetic Resonance Imaging (MRI) is based on changes in behavior of water molecules in an external magnetic field of the scanner. The behavior is dependent on a water concentration in the tissue, on a concentration of ions and organic compounds in the body fluids, on pH, on temperature etc. Presence of paramagnetic compounds dramatically changes the behavior of water molecules and thus, most of commercially applied contrast agents are based on gadolinium(III) ion. This ion is (as well as other lanthanide and transition metal ions) toxic and thus, it is necessary to apply it in form of a complex. Complexation of Gd(III) ion unfortunately decreases its contrasting ability. Therefore, suitable complex must exhibit a sufficient stability on one hand and a sufficient transfer of a magnetic information between the metal ion and the water molecules of the bulk. A design of suitable ligands is in the center of the interest of many laboratories around the world. One way of the contrast agent improvement is attachment of a complex to macromolecular carrier. Another group of MRI contrast agents is based on superparamagnetic properties of nanocrystaline iron oxides. Today, new class of contrast agents selectively binding on a target tissue (so called “Targeted agents”) is developed. These contrast agents decrease applied doses without decreasing of the contrast.
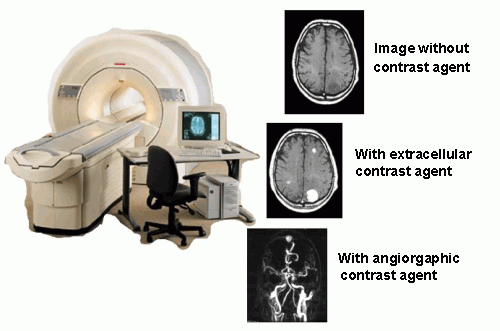
Contrast agents for MRI tomography

Today used MRI contrast agents are based mostly on gadolinium(III) ion. Due to its toxicity and high applied doses (1 – 10 g per one investigation), the ion must be bound in a thermodynamically and kinetically stable complex. An efficiency of a contrast agent is expressed as a relaxivity (relaxation rate of water protons in a 1mM solution of gadolinium). The relaxivity (and efficiency of the contrast agent) is dependent on many parameters. The most important parameters are a magnetic field of an MRI scanner, temperature, a number of water molecules directly coordinated to the Gd(III) ion, exchange rate of the coordinated water molecules τM, rotation correlation time τr, electronic relaxation parameters of the metal ion etc. Lanthanide(III) complexes can mostly be characterized by NMR, EPR and luminescence spectroscopy. The coordination number of Gd(III) ion is 8 or 9 in dependence on steric requirements of ligands. In MRI, the ligands containing eight donor groups are used. Ligands used as commercially applied MRI contrast agents are derived from polyaminocarboxylates DOTA a DTPA). The exchange of the water molecule between the coordination sphere and the bulk solvent is main way how the magnetic information is transferred. Absence of a water molecule in the first coordination sphere results in reduction of the magnetic information transfer and in a decrease of contrast agent efficiency. Contrary, presence of two coordinated water molecules decreases stability of the complex. Furthermore, flexibility of such a coordination sphere allows substitution of the coordinated water molecules by biogenic ligands (acetates, carbonates, aminoacids, etc.) resulting again in the contrast ability decrease. The exchange rate of the coordinated water molecules is one of the most important parameters, which can be chemically tuned through choice of suitable ligand. To reach the maximum efficiency, the residence time (τM) of the water molecule in the coordination sphere should be 10–20 ns for magnetic fields used nowadays. Generally, the water exchange rates of the current contrast agents are too slow. Another parameter, which can be chemically modified, is size of a molecule and its tumbling rate (rotation corellation time τr). Bigger molecules show better MRI properties. The increase of the rotation correlation time is usually reached by attachment of a low-molecular weight complex to a macromolecular carrier (e.g. dendrimer). The dependency of the relaxivity on the water residence time and on the rotation correlation time shows a mountain-like shape. The efficiencies of the today-applied contrast agents are close to the root of the mountain (see arrow in the picture) and we shall climb up.
Radiopharmaceutical applications
The radioisotopes are used for diagnosis as well as for a treatment of mostly cancerous diseases. The most commonly used isotope is 99mTc. Technetium ions form stable complexes in low oxidation states with nitrogen-, sulphur- or trivalent phosphorus-containing ligands. On the other hand, there are only few suitable ligands for many radioisotopes of other metal isotopes (e.g. 64/67Cu,68Ga, 111In, 90Y, 153Sm, 166Ho, 177Lu, 201Tl, 212Pb, 212/213Bi etc.). Many of them are interesting due to their excellent physical properties, a good accessibility and/or a low price. Suitable ligands for the metal radioisotopes must form thermodynamically and kinetically stable complexes. It is necessary to avoid any decomposition of such complex in organism and thus, the in vitro stabilities of the complexes are intensively studied (as well as study of complexation/decomplexation equilibria under chemical and radiochemical conditions). The complexes should exhibit a suitable pharmacokinetic behavior (the complex should bind fast and selectively in a desired tissue; an excess of the complex is swiftly excreted, whereas the urine excretion is preferred). Beside it, a complexation process should be fast, as the half-lives of the applied radioisotopes are in range of minutes to hours and thus, process of the preparation of isotope, complexation, purification and the application is time-limited. Due to a negative effect of radioactivity on organisms, “targeted” ligands are developed. These ligands are selectively bound mainly to a target tissue, what reduces negative effects of radiation on rest of body.
Stability of the complexes
 Complexes of transition metal and lanthanide ions for biomedical applications should exhibit a high kinetic and
thermodynamic stability to avoid their decomposition and an intoxication of the organism. For radiopharmaceutical
applications, it is necessary to reduce, as much as possible the time of the complex preparation and the complexation
rate becomes an important parameter. New generations of radiopharmaceuticals often contain some pH and temperature sensitive
components (peptides, polysacharides) limiting the complexation conditions. Under “chemical” conditions, kinetics of
the complexatin/decomplexation process is usually studied by the UV/vis spectroscopy. Beside the formation rate,
reaction mechanisms of the processes can be usually determined. As a complexation with polydentate ligands shows very
often a complicated mechanism, such knowledge is crucial for a design of new, more efficient ligands.
Complexes of transition metal and lanthanide ions for biomedical applications should exhibit a high kinetic and
thermodynamic stability to avoid their decomposition and an intoxication of the organism. For radiopharmaceutical
applications, it is necessary to reduce, as much as possible the time of the complex preparation and the complexation
rate becomes an important parameter. New generations of radiopharmaceuticals often contain some pH and temperature sensitive
components (peptides, polysacharides) limiting the complexation conditions. Under “chemical” conditions, kinetics of
the complexatin/decomplexation process is usually studied by the UV/vis spectroscopy. Beside the formation rate,
reaction mechanisms of the processes can be usually determined. As a complexation with polydentate ligands shows very
often a complicated mechanism, such knowledge is crucial for a design of new, more efficient ligands.
 The thermodynamic stability of the complexes and acid-base properties of the ligands are followed by many methods. The most common methods
are pH-metry (follow changes of proton concentration caused by the complexation process), NMR spectroscopy (changes of
chemical shift of an observed nucleus as result of geometric and electronic changes during complex formation) or UV/vis
spectroscopy (color changes resulting from changes in coordination sphere of the metal ion or ligand conformation upon
coordination). The methods can be used not only for a determination of complex thermodynamic stability, but also for
identification of a complex composition in solution. To get better information about the structure of the studied
complexes, spectroscopic methods (NMR, EPR, absorption and luminescent spectroscopies, etc.) and X-ray diffraction
analysis should be used.
The thermodynamic stability of the complexes and acid-base properties of the ligands are followed by many methods. The most common methods
are pH-metry (follow changes of proton concentration caused by the complexation process), NMR spectroscopy (changes of
chemical shift of an observed nucleus as result of geometric and electronic changes during complex formation) or UV/vis
spectroscopy (color changes resulting from changes in coordination sphere of the metal ion or ligand conformation upon
coordination). The methods can be used not only for a determination of complex thermodynamic stability, but also for
identification of a complex composition in solution. To get better information about the structure of the studied
complexes, spectroscopic methods (NMR, EPR, absorption and luminescent spectroscopies, etc.) and X-ray diffraction
analysis should be used.

Targeting
The targeting (specific binding to the target tissue) is a method resulting in a reduction of applied pharmaceutical doses, while effect of contrast agent or of pharmaceutical is maintained or even increased. This can be reached by the attachment of a biologically active molecule. This molecule shows high affinity and selectivity to the target tissue/receptors. Upon application, the compound is bound to the target tissue/receptors. The biological active compound can be a peptide (e.g. modified hormones such as somatostatin analogues), polysacharides, monoclonal antibodies or their fragments, compounds bound through hydrophobic interactions or bis(phosphonates) for calcified tissues. The spacer connecting a pharmaceutical with a targeting group plays also important role, as it can dramatically change pharmacokinetics of the studied compound.

| PET (Positron Emission Tomography) image of a mouse. Application of contrast agent
selectively bound on the bone tissue prepared in our group |