ACTA UNIVERSITATIS CAROLINAE - BIOLOGICA 3O, (1986): 281-286
ISSN 0001- 7124
JAROSLAV FLEGR1, ,JIŘÍ ČERKASOV1, JAROSLAV KULDA1, APOLENA ČERKASOVOVÁ, JITKA ŠTOKROVÁ 3
Faculty of Science, Charles University, Praguel
Postgraduate Medical and Pharmaceutical Institute, Prague2
Institute of Molecular Genetics, Prague3
Author,s address: Department of Physiology, Faculty of Science, Charles University, Vinicná 7, 128 44 Prague 2, Czechoslovakia.
Abstract: Nucleic acids extracts of T. vaginalis prepared by guanidine hydrochloride method were analyzed by agarose electrophoresis. Six of sixteen examined strains contained considerable amount of double stranded RNA. No correlation was observed between presence of dsRNA and metronidazole sensitivity or virulence of the examined strains. Electrophoretic pattern of the dsRNA suggested presence of six distinct populations of dsRNA at least. After deferential centrifugation the main portion of the dsRNA was found in the large granule fraction of the cell homogenate suggesting association of dsRNA with sedimentable particles.
Key words: Trichomonas vaginalis, dsRNA, cell fractionation, virus.
INTRODUCTION
Occurrence of dsRNA in T. vaginalis cell has been reported recently (WANG and WANG, 1985). The authors have shown that nucleic acids extracts from 33 of 35 strains examined contained 5.5 kb linear molecules of dsRNA. The amount of the dsRNA was about 10 % of the amount of DNA. Hybridization experiments revealed that DNA of all examined trichomonas strains possessed sequences homologous to dsRNA irrespectively of the presence or absence of dsRNA in the cell. The results of hybridization experiments and unusual properties of the two strains lacking dsRNA (both were metronidazole resistant) led the authors to assume that the dsRNA is intrinsic to typical T. vaginalis cell and is not of viral origin.
In this paper we report on the occurrence of dsRNA in different T. vaginalis strains, on the length distribution profile of the molecules and on their subcellular localization. Our data indicate that the viral origin of the T. vaginalis dsRNA cannot be excluded.
MATERIALS AND METHODS
Trichomonads
All strains excepting A-1 (Vienna, Meingassner) were isolated in Prague. The cultures were axenized, assayed for virulence and stored as cryostabilates in liquid nitrogen.
Trichomonads for isolation of dsRNA were cultivated in Diamond's TYM medium (Diamond, 1957) pH 6.0 without agar, with 10 % inactivated horse serum. Cells were harvested in the late logarithmic phase of growth and washed with 0.8 % NaCl.
Cell fractionation
Cells were homogenized in the isotonic medium (1M) (0.225 M sucrose, 0.01 M KH2PO4, 0.02 M Tris, 0.005 M MgCl2, 0.001 M EDTA, pH 7.2) and fractionated by differential centrifugation according to ČERKASOV et al. (1978). Large granule fraction (LGF) prepared from about 109 cells was resuspended in 5 ml of the IM and laid in 1 ml aliquots on the top of 10 ml discontinual Nycodenz gradient (l.l3-1.30 g/ml in IM). Gradients were centrifuged on Spinco Beckman centrifuge, Model L2-6513 using the rotor S'V 41 (40 008 rpm, 1.5 h., 4o C).
Isolation and electrophoresis of nucleic acids.
Nucleic acids were extracted eithr from 4 ml cell suspension (l.l0s cells/ml) or 0.2 ml subcellular fractions (0.7-2.1 mg/ml protein) in 4 M guanidine hydrochloride (Pramanick et al., 1975). Before electrophoresis, some extracts were treated with DNase 1 (30 .g/ml, 20o C, 30 min.).
Nucleic acids extracts were electrophopresed in 0.8 % agarose according to MANIATIS et al. (1983). After electrophoresis the gels were stained by 0.0005 % solution of ethidium bromide in H20 or by 0.00001 % DAPI (4'-6-diamidino-2-pheny-lindole) or 0.003 % acridine orange in 0.01 M phosphate buffer pH 7.1. After staining some gels were treated by DNase 1 (50 ug. ml, 2 h, 37o C) in the medium incubation buffer (Maniatis et al.,1983).
Electron microscopy
Preparation of specimens of dsRNA for electron microscopy, visualization of molecules, measurement of contour length and processing of numerical data were carried out as described elsewhere (Davis et al.,1971; Hochmanová et al.,1982).
Chemicals
Deoxyribonuclease 1 (450u/mg) was obtained from Koch-Light Laboratories, England; Nycodenz from Nyegard & Co, Norway; Ethidium bromide from Sigma. USA. All other chemicals were of analytical grade.
RESULTS
Agarose electrophoresis of T. vaginali.s nucleic acids extracts revealed six distinct bands of dsRNA (Figs 1, 2). The RNA nature of these bands was proved by their resistance to DNase and by their failure to stain with DAPI (DNA specific fluorescence probe). Bluish fluorescence with acridine orange and electron microscopy (Fig. 3) indicated the double stranded character of this RNA.
Fig. 1. Fig. 2.
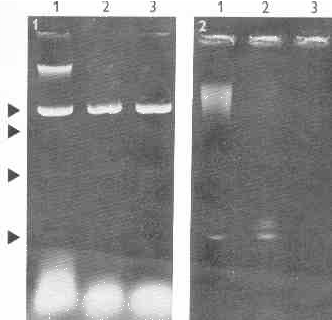
Fig.1. Electrophoresis of nucleic acids of T. vaginalis. Lane 1- nucleic acids extract of T. vaginalis strain A1, lanes 2, 3 - the same extract incubated with DNase 1 (30 .g/ml, medium incubation buffer of Maniatis). Four dsRNA bands are present (arrows).
Fig. 2. Electrophoresis of nucleic acids of T. vaginalis. Lane 1- nucleic acids extract of T. vaginalis strain A1, lanes 2, 3 - the same extract after DNase 1 treatment. Electrophoresis was performed three times longer than on Fig. 1 (10 h.), consequently DNA and major dsRNA bands only are present. It is evident that the dsRNA band consists of three distinct populations of molecules at least.
Fig. 3.
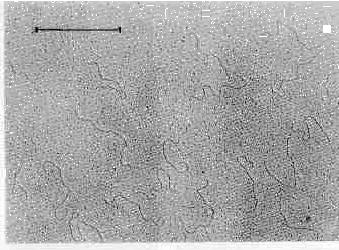
Fig. 3. Electron microscopy of dsRNA. dsRNA molecules were visualized as described by Hochmanová et al. (1982). Under nondenaturating conditions of the aqueous protein monolayer technique, the main component of DNase treated nucleic acids extracts were linear molecules of maximum length about 1.7 um. Bar: 1 um.
After differential centrifugation of the cell homogenate of the T. vaginalis strain A1 the main portion of dsRNA was found in fraction of large granules (LGF) (Fig. 4). When the LGF was subjected to centrifugation on discontinual Nycodenz gradient, the dsRNA banded in fractions 2 - 5 with the peak in the fractions 3 and 4 (1.15 and 1.17 g/ml) (Fig. 5). It was undetectable in the remaining fractions although they contained bands of subcellular components. The distribution of the dsRNA in the gradient suggested its association with a distinct component present in the T. vaginalis cells.
Fig. 4 Fig. 5
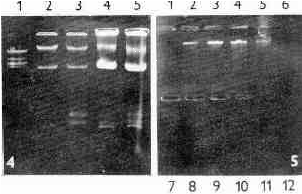
Fig. 4. Electrophoresis of nucleic acids extracts obtained from two fractions of T. vaginalis A1 homogenate. Lane 1 - Eco RI digest of DNA of phage lambda, lanes 2, 3 - extract of nuclear fraction, lanes 4, 5 - extract of large granule fraction. Fractionation of cell homogenate according to Čerkasov et al., (1978).
Fig. 5. Electrophoresis of nucleic acids extracts from Nycodenz density gradient fractions of T. vaginalis A-1. Large granule fraction of cell homogenate prepared according to Čerkasov et al., (1978) was fractionated by Nycodenz density gradient. Lane 1 - 1.05 g/ml, lane 12 - 1.30 g/ml. Note the dsRNA bands in lanes 2-5 showing the peak in lanes 3 and 4 (1.151 and 1.169 g/ml, respectively).
Different strains of T. vaginalis were screened for the presence of dsRNA (Tab.1) Detectable amount of dsRNA was found in six of sixteen strains examined. Dat; presented in the table do not indicate a correlation between the presence of dsRNA and virulence or resistance to m metronidazole of the assayed organism.
Table l. Some properties of dsRNA plus and dsRNA minus strains of T. vaginalis. (Unless indicated
otherwise the strains were isolated from different female patients in Prague).
Strain Resistance to metronidazole Virulence* dsRNA
A-1 ** - not determined +
TVl0-02 - mild +
TVl0-02-MR-100*** + not determined +
TV 10-02AT * * * * - not determined +
TVl7-48 - high -
TV 5-27 - moderate -
TVl4-85 - moderate -
TV30-32 - moderate -
TV 7-37 - high -
TV84-00 - high -
TV73-87 - moderate +
TV79-49 - high +
TV71-96 - moderate +
TV85-08 - moderate +
TV67-77 - mild -
MRP-2 - not determined -
* virulence was estimated on the basis of clinical and histopathological manifestations in female patients, by sub-
cutaneous mouse assay and by cumulative mortality of mice inoculated intraperitoneally
** clinical isolate from Vienna, Austria obtained from J. G. Meingassner
*** in vitro developed metronidazole resistant strain (anaerobic resistance) derived from TV10-02
**** line of the TV10-02 strain maintained in active culture over 200 transfers (medium TYM,37o C)
DISCUSSION
Results presented in this paper confirm the presence of dsRNA in certain strains of T. vaginalis. In contrast to findings of WANG and WANG (1985) a minor part of material only (6 of 16 examined strains) contained the dsRNA. Further, our result did not confirm suggested correlation between the presence of dsRNA and susceptibility to metronidazole, as seven drug susceptible strains examined contained no detectable dsRNA.
Electrophoretic pattern of dsRNA demonstrated several distinct populations of molecules differing in length from the molecules of the major band. Such configuration is reminiscent of segmental genome of dsRNA viruses.
Distribution of dsRNA after differential centrifugation of the T. vaginalis homogenate indicated association of the dsRNA with a sedimentable particle.
These results are suggestive of viral origin of the T. vaginalis dsRNA. WANG and WANG (1985), however, failed to demonstrate presence of virus like particles by electron microscopy. In addition, presence of sequences homologous to dsRNA in DNA of the host cell, as demonstrated in trichomonads by WANG & WANG (1985), has not been observed in any dsRNA viruses so far*. Further studies are necessary to understand the origin and biological significance of the T. vaginalis dsRNA.
REFERENCES
ČERKASOV, J., A. ČERKASOVOVÁ, J. KULDA, D. VILHELMOVÁ, 1978: Respiration of hydrogenosomes
of Trichomonas foetus: I. ADP dependent oxidation of malate and pyruate. J. Biol. Chem. 253;1207-1214.
DAVIS, R. W., M. SIMON, N. DAVIDSON, 1971: Electron microscope heteroduplex methods for mapping regions of base sequence homology in nucleic acids. Methods Enzymol. 21 D: 413 -428.
DAMOND, L. S.,1957: The establishment of various trichomonads of animals and man in axenic cultures. J. Pcrrasitol. 43: 488 -490.
HOCHMANNOVÁ, .J., .J. NEŠVERA, J. ŠTOKROVÁ, J. STRÁNSKÝ, 1982: Molecular and genetic properties
of plasmid R 485 conferring resistance to sulfonamides. J. Gen. Microbiol.128: 529.
MANIATIS, T., E. F. FRITSCH, J. SAMSROOK,1983: A laboratory manual, Molecular Cloning, Cold
Spring Harbor Laboratory Press, NY.
PRAMANICK, D., J. FORSTOVÁ, L. PIVEC, 1975: 4 M Guanidine hydrochloride applied to the isolation RNA from different sources. FEBS Letters 62: 488-490.
WANG, A. L., C. C. WANG, 1985: A linear double stranded RNA in Trichomonas vaginalis. J. Biol. Chem. 260: 3 697-3 702.
* Editors' addendum: Subsequent studies of WANG and WANG did not confirm presence of sequences homologous to dsRNA in the nuclear DNA of T. vaginalis. On the other hand, the viral origin of the T. vaginalis dsRNA has been confirmed by recent investigations. See references: WANG, A. L. and C. C. WANG, 1986: The double stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc. Nat. Acad. Sci. USA 83: 7 956-7 961. FLEGR, J., J. ČERKASOV, J. KULDA, J. TACHEZY AND J. ŠTOKROVÁ, 1987: The dsRNA of Trichomonas vaginalis is associated with virus-like particles and does not correlate with metronidazole resistance. Folia Microbiol. 32: 345-348.