1. Analysis of Src signaling using SrcFRET biosensor
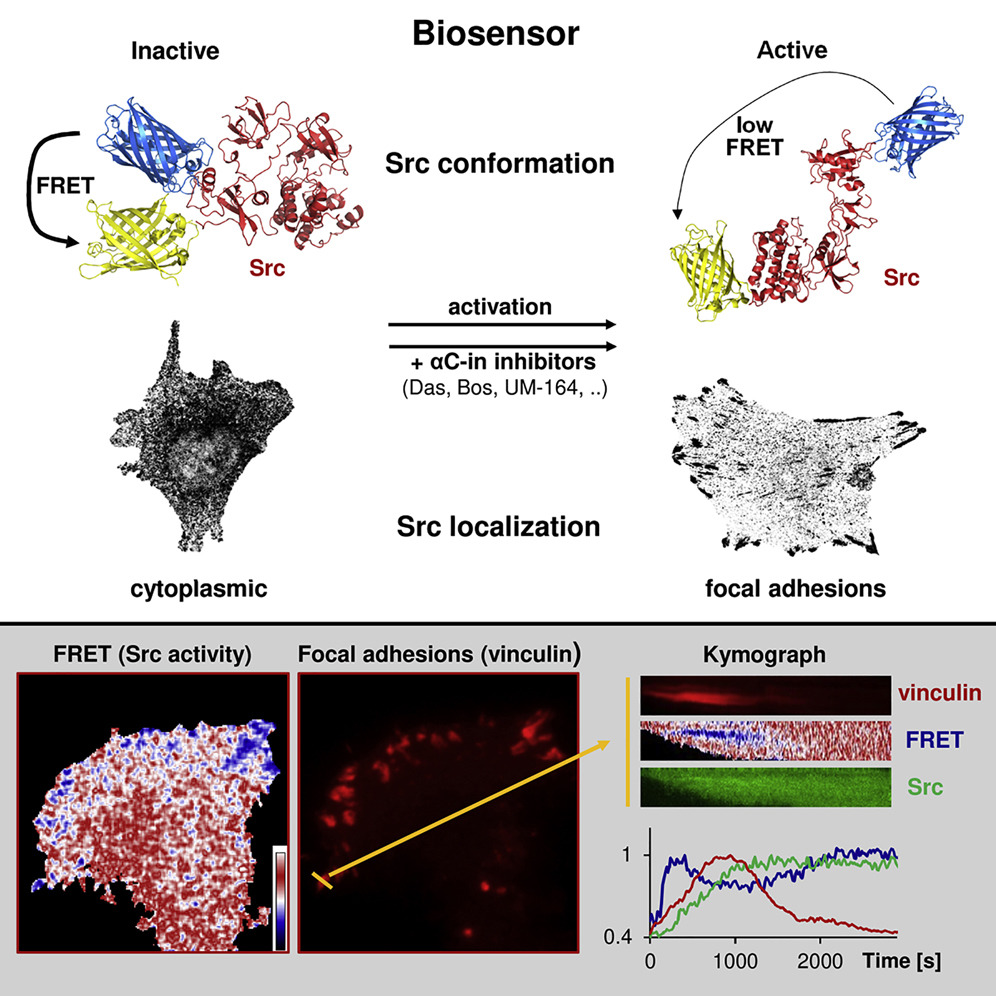
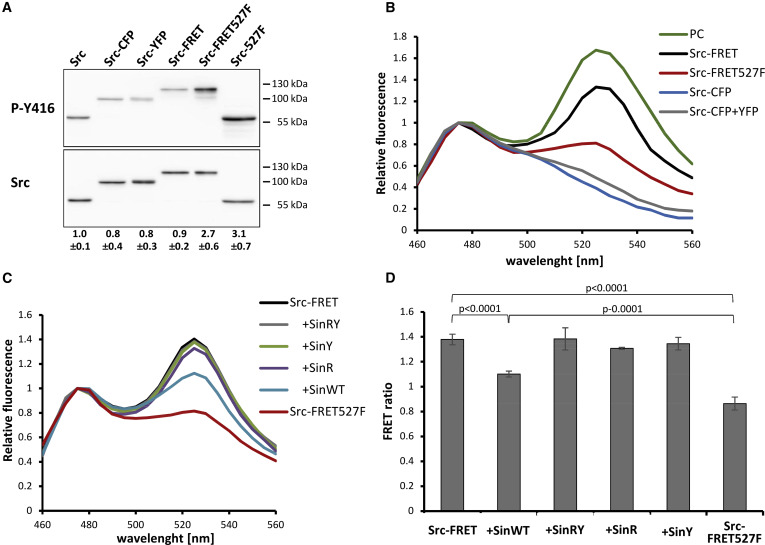
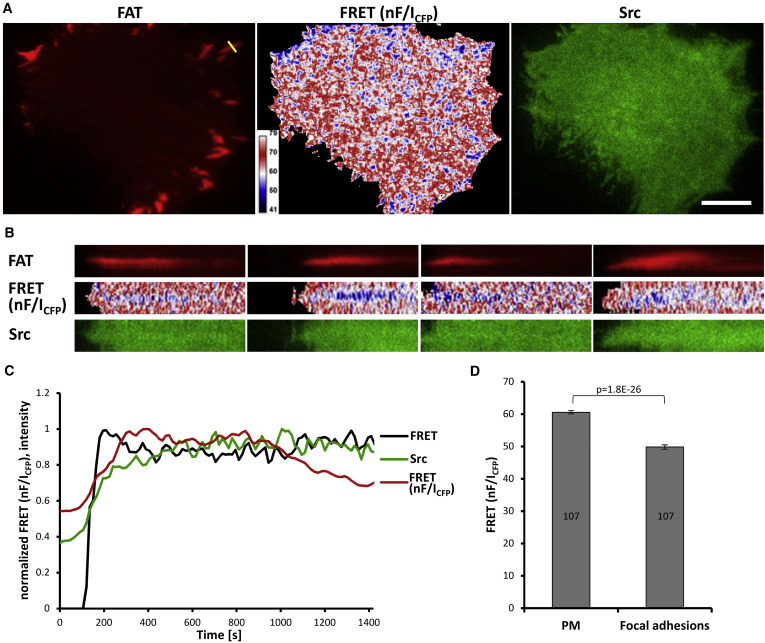
Src kinase plays an important role in a multitude of fundamental cellular processes and is often found deregulated in tumors. Active Src adopts an open conformation, whereas inactive Src is characterized by a very compact structure stabilized by inhibitory intramolecular interactions. Taking advantage of this spatial regulation, we constructed a fluorescence resonance energy transfer (FRET)-based Src biosensor and analyzed conformational changes of Src following Src activation and the spatiotemporal dynamics of Src activity in cells (Koudelkova et al, 2019). We found that activatory mutations either in regulatory or kinase domains induce opening of the Src structure. Surprisingly, we discovered that Src inhibitors differ in their effect on the Src structure, some counterintuitively inducing an open conformation. Finally, we analyzed the dynamics of Src activity in focal adhesions by FRET imaging and found that Src is rapidly activated during focal adhesion assembly, and its activity remains steady and high throughout the life cycle of focal adhesion and decreases during focal adhesion disassembly.
Related publications:
Phosphorylation of tyrosine 90 in SH3 domain is a new regulatory switch controlling Src kinase. Koudelková L, Pelantová M, Brůhová Z, Sztacho M, Pavlík V, Pánek D, Gemperle J, Talacko P, Brábek J, Rösel D. Elife. 2023 Jul 10;12:e82428. DOI
Src kinase: Key effector in mechanosignalling. Koudelková L, Brábek J, Rosel D. 2021. Biomolecules. 131:105908. DOI
Novel FRET-Based Src Biosensor Reveals Mechanisms of Src Activation and Its Dynamics in Focal Adhesions. Koudelková, Lenka, Andreea Csilla Pataki, Ondřej Tolde, Vojtech Pavlik, Max Nobis, Jakub Gemperle, Kurt Anderson, Jan Brábek, and Daniel Rosel. 2019. Cell Chemical Biology 26 (2): 255-268.e4. DOI
2. Analysis of mechanosensory role of p130Cas/BCAR1
Cells sense these mechanical cues through specialized mechanosensory proteins. One of such mechanosensory proteins is p130Cas/BCAR1. P130Cas is a major substrate of Src proto-oncogene, plays an important role in oncogenic transformation mediated by the v-crk and v-src oncogenes and increased levels of its human ortholog, BCAR1, are associated with exacerbated prognosis in breast cancer patients. Our goal is to analyze mechanosensory properties of p130Cas and mechanotransduction in focal adhesions and invadopodia. To achieve these goals, we analyze, among others, the cellular response to mechanical stress and monitor tension within the adhesive structures using FRET-based tension biosensors.
Related publications:
The Interaction of P130Cas with PKN3 Promotes Malignant Growth. Gemperle, Jakub, Michal Dibus, Lenka Koudelková, Daniel Rosel, and Jan Brábek. 2019. Molecular Oncology 13 (2): 264–89. DOI
Structural Characterization of CAS SH3 Domain Selectivity and Regulation Reveals New CAS Interaction Partners. Gemperle, Jakub, Rozálie Hexnerová, Martin Lepšík, Petr Tesina, Michal Dibus, Marian Novotný, Jan Brábek, Václav Veverka, and Daniel Rosel. 2017. Scientific Reports 7 (1): 8057. DOI
The Role of Focal Adhesion Anchoring Domains of CAS in Mechanotransduction. Braniš, Jaroslav, Csilla Pataki, Marina Spörrer, Richard C Gerum, Astrid Mainka, Vladimir Cermak, Wolfgang H Goldmann, Ben Fabry, Jan Brabek, and Daniel Rosel. 2017. Scientific Reports 7: 46233. DOI
Mechanosensors in Integrin Signaling: The Emerging Role of P130Cas. Janoštiak, Radoslav, Andreea Csilla Pataki, Jan Brábek, and Daniel Rösel. 2014. European Journal of Cell Biology 93 (10–12): 445–54. DOI
CAS Directly Interacts with Vinculin to Control Mechanosensing and Focal Adhesion Dynamics. Janoštiak, Radoslav, Jan Brábek, Vera Auernheimer, Zuzana Tatárová, Lena A Lautscham, Tuli Dey, Jakub Gemperle, et al. 2014. Cellular and Molecular Life Sciences : CMLS 71 (4): 727–44. DOI
Tyrosine Phosphorylation within the SH3 Domain Regulates CAS Subcellular Localization, Cell Migration, and Invasiveness. Janoštiak, Radoslav, Ondřej Tolde, Zuzana Brůhová, Marian Novotný, Steven K Hanks, Daniel Rösel, and Jan Brábek. 2011. Molecular Biology of the Cell 22 (22): 4256–67. DOI
3. Crosstalk of PKN3 and Rho in signaling
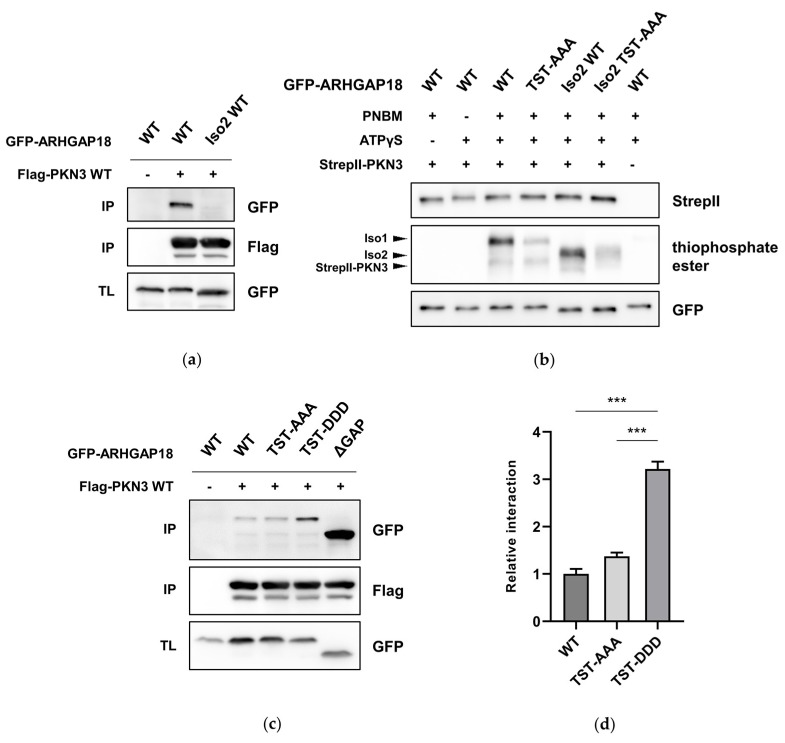
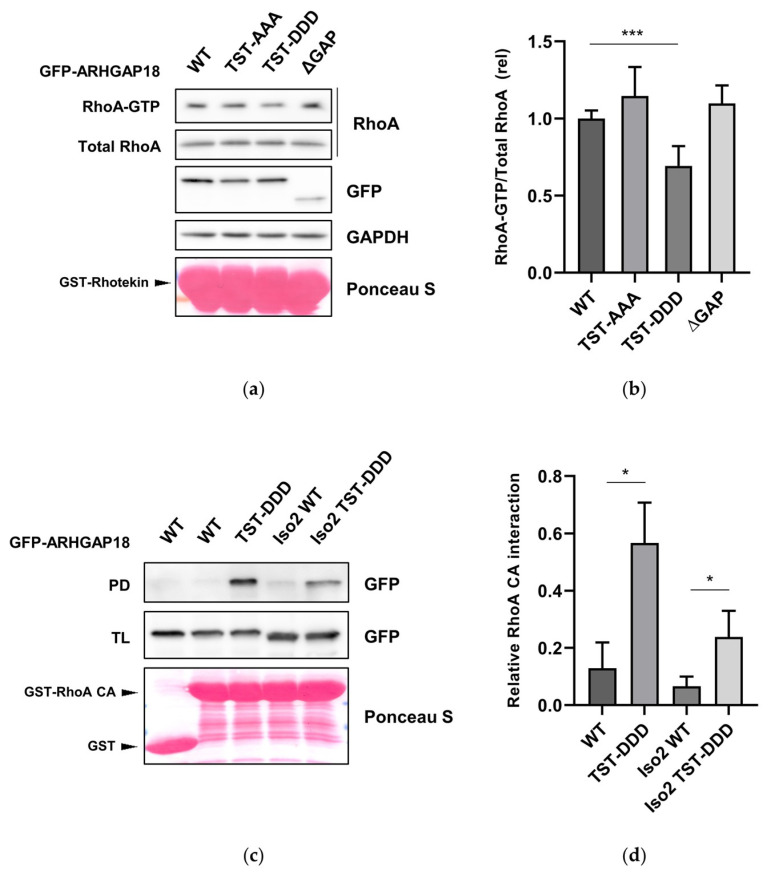
Protein kinase N3 (PKN3) is a serine/threonine kinase implicated in tumor progression of multiple cancer types, however, its substrates and effector proteins still remain largely understudied. Recently, in Dibus et al. we presented strong evidence of PKN3-ARHGAP18 interaction, which is increased upon ARHGAP18 phosphorylation by PKN3, leading to enhanced GAP domain activity. This reveals a negative regulation of RhoA activity by PKN3-ARHGAP18.
Related publications:
A Screen for PKN3 Substrates Reveals an Activating Phosphorylation of ARHGAP18. Dibus, Michal, Jan Brábek, and Daniel Rösel. 2020. International Journal of Molecular Sciences 21 (20). DOI