Siglec-7 and Immune Evasion – Our Research Featured on the Faculty Website
Our team’s research has been highlighted in the news section of the Faculty of Science, Charles University!
In the article entitled “How Unwanted Visitors Tricked the Immune System“, the study describes how tumour cells and pathogens evade immune surveillance by mimicking host surface glycan structures.
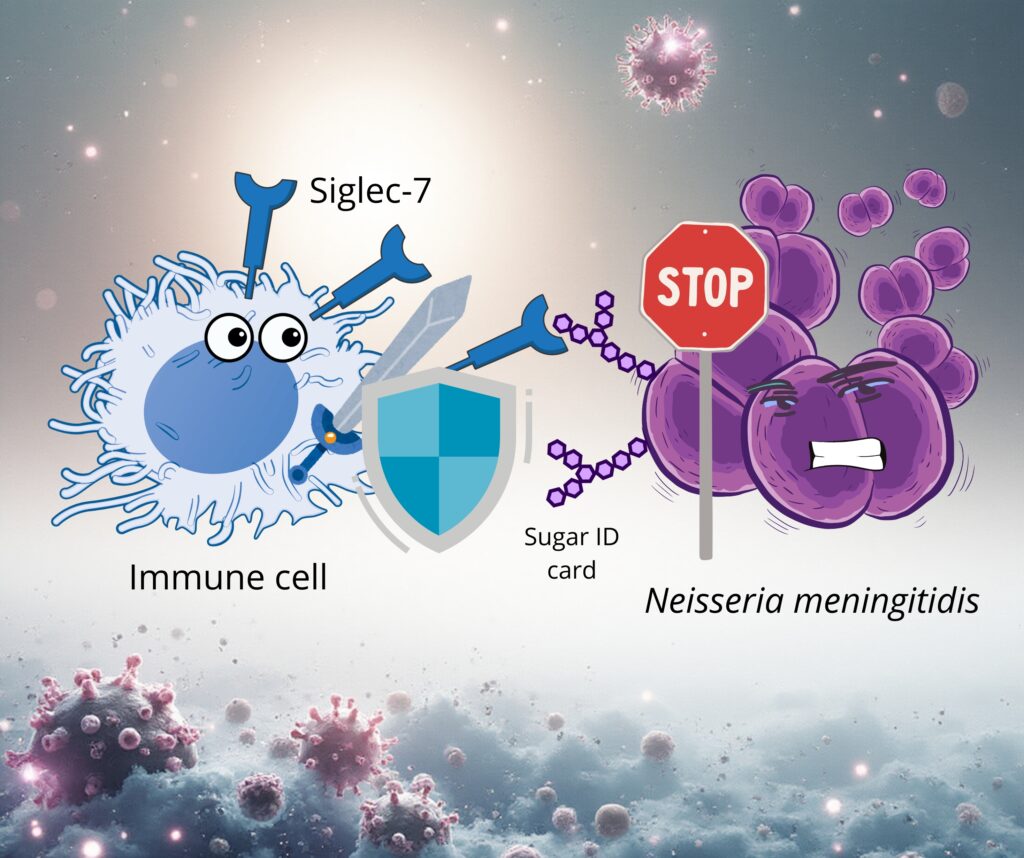
These invaders exploit sialic acid–decorated “sugar ID cards” to engage the inhibitory receptor Siglec-7 on natural killer (NK) cells. When Siglec-7 is engaged, it sends a “do not attack” signal, allowing the disguised cells to evade immune elimination.
In our lab, we characterised the extracellular domain of Siglec-7, demonstrated the presence of a stable dimeric form, and developed a method for producing doubly ¹³C/¹⁵N-labelled protein in mammalian cells—enabling detailed NMR structural studies. Thanks to these studies, it was identified that the tumour-associated ganglioside GD3 binds directly to Siglec-7 and that arginine 124 is a key residue mediating this interaction.