Antigen presenting cells
The cornerstone of the immune system lies in its remarkable ability to differentiate between harmless non-self entities and harmful intruders, as well as self-derived structures and their altered, pathogenic counterparts. While T-cells are the critical effector cells responsible for eliminating pathogens or establishing tolerance towards commensals and self-derived structures, the pivotal point where fate decisions in the immune response are made is at the intersection of antigen-presenting cells and their intricate communication with T-cells.
Professional antigen-presenting cells, which exist in various tissues, have diverse ontogenetic origins, and fulfill distinct physiological roles, share the unique capacity to acquire, process, and present antigens within the context of MHCII to CD4+ T-cells. These cells efficiently trigger, guide, or hinder T-cell responses. Our particular focus centers on the “non-classical” MHCII-positive antigen-presenting cells, such as epithelial cells and innate lymphoid cells, and their role in regulating adaptive immunity.
We firmly believe that advancing our understanding of these immune pathways will pave the way for more precisely targeted therapies, especially in the context of chronic diseases characterized by disrupted tissue homeostasis due to pathogens or autoimmune responses.

MHCII+ Innate Lymphoid Cells
Innate lymphoid cells (ILCs) play a crucial role in swiftly modulating immune responses, maintaining homeostasis, and preserving the integrity of epithelial barriers through the rapid release of cytokines. Surprisingly, recent investigations have unveiled various ILC subsets that possess the ability to express MHCII and engage in antigen presentation. These unique cells are capable of leveraging their antigen-presenting capacity to influence and regulate adaptive immune responses.
Our research focuses on unraveling the processes employed by MHCII-expressing ILCs to acquire antigens, effectively present them, and thus regulateT-cell responses. We explore these phenomena not only in steady-state conditions but also in scenarios disrupted by factors such as pathogenic infections or autoimmune reactions.
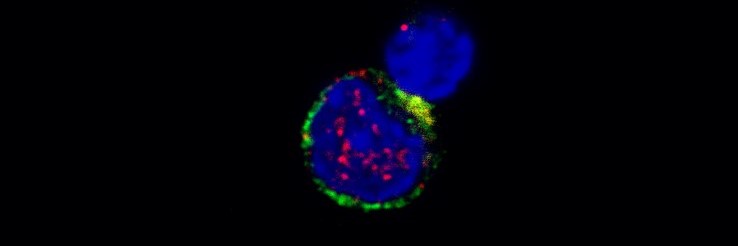
Medullary thymic epithelial cells
Medullary thymic epithelial cells (mTECs) play a pivotal role in guiding the development of T-cells in the thymus and preventing autoimmune reactions triggered by the recognition of self-antigens in the peripheral immune system. They accomplish this by possessing the remarkable capability to produce nearly the entire host’s coding genome and present it as antigens on their MHC molecules to developing T-cells. Self-reactive T-cell clones are effectively screened out or transformed into T-regulatory cells, contributing to immune tolerance.
The antigens expressed by mTECs can also be transferred to other antigen-presenting cells in a coordinated manner. Our research is primarily focused on understanding the mechanisms governing mTECs’ capacity to coordinate the transfer of antigens within the thymus and how distinct thymic antigen-presenting cells work together to enforce immune tolerance.
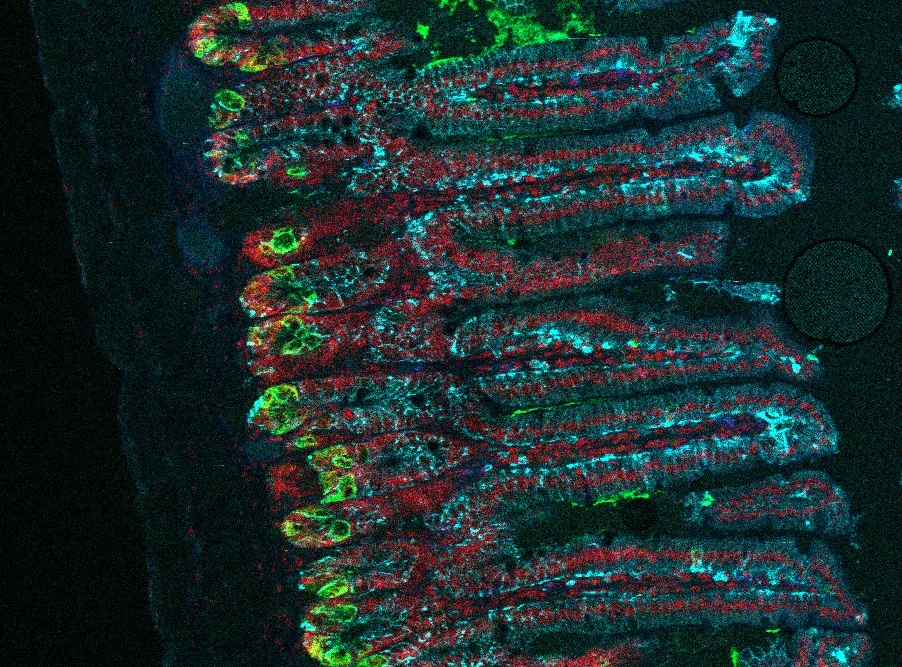
Intestinal epithelial cells
Intestinal epithelial cells (IECs) serve as the boundary delineating the intestinal lumen and play a crucial role in the digestive process. They are the first line of defense separating the body from the intestinal lumen. In addition to their digestive functions, IECs also act as key players in the innate immune response by producing various antimicrobial substances. However, their role in initiating and regulating adaptive immune responses has only recently come to light.
One noteworthy discovery has been the recognition of IECs’ ability to express MHCII molecules, which has a significant impact on the regulation of immune responses within the intestines. Our research is centered on understanding the mechanisms that trigger the expression of MHCII on IECs, as well as their capacity to utilize MHCII for antigen presentation, thereby shaping T-cell responses to intestinal (patho)bionts. We are also delving into the molecular mechanisms employed by IECs to acquire antigens for presentation on MHCII molecules.