Two New Collaborative Papers Reveal Mechanisms of Siglec-7 Ligand Binding
Our laboratory has contributed to two newly published studies investigating the role of Siglec-7, an inhibitory receptor expressed on natural killer (NK) cells, in immune regulation through its interactions with glycan ligands in both cancer and bacterial infection contexts.
In the first study, researchers examined how Siglec-7 recognises the tumour-associated ganglioside GD3, a disialylated glycosphingolipid highly expressed on cancer cells such as melanoma and pancreatic carcinoma.
Using structural biology, NMR, and computational modelling, the team revealed how the conformation and flexibility of ganglioside ligands affect their binding to Siglec-7. While GD3 exhibits a strong binding affinity, more flexible derivatives, such as Gb3, bind less effectively due to entropic penalties. These findings enhance our understanding of immune evasion in cancer and may lead to the development of new glycan-based therapeutic strategies.
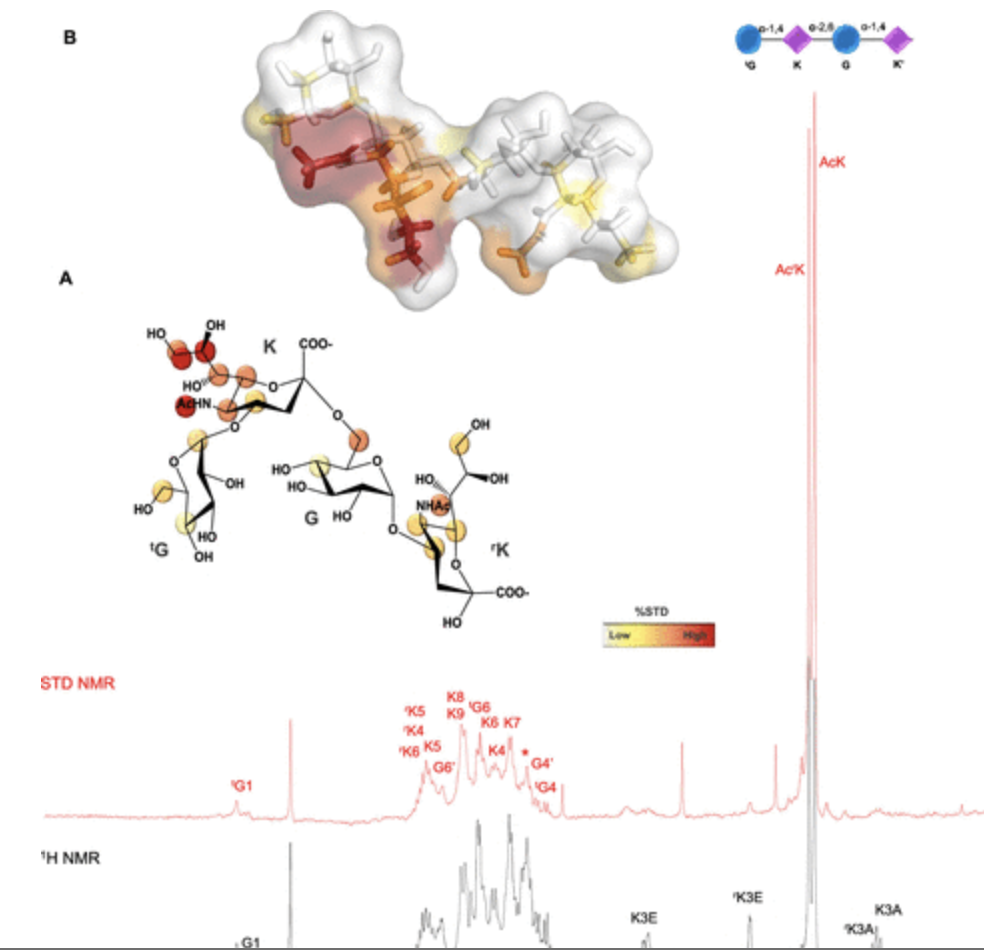
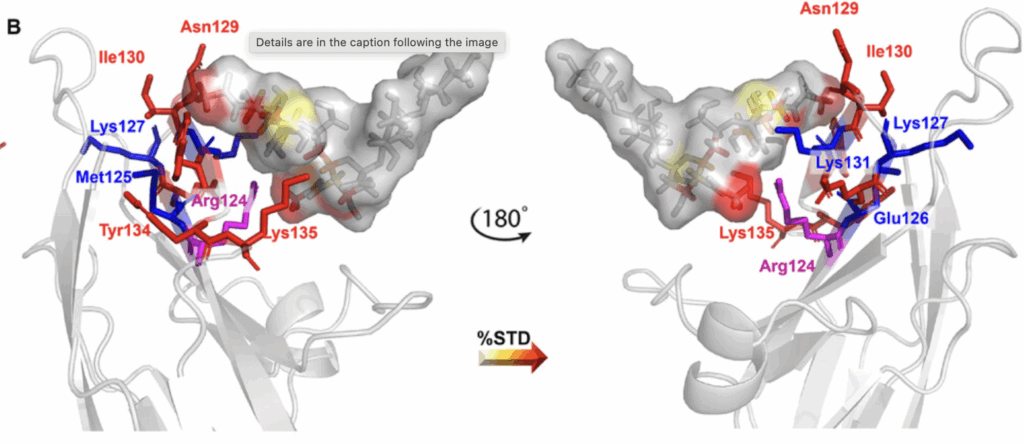
The second study explored how the sialylated capsular polysaccharide (CPS) of Neisseria meningitidis serogroup Y (Men-Y) interacts with Siglec-7.
Employing ELISA, fluorescence binding assays, NMR spectroscopy, and computational analysis, the research demonstrated that the specific α-2,6-linked sialic acid presentation within the CPS is key to binding Siglec-7. Functional assays further suggested that Men-Y CPS can modulate immune cell responses via Siglec-7 engagement, shedding light on a novel bacterial immune evasion mechanism.