New paper is out! Find about NKp46 glycosylation and Candida glabrata interaction
We are proud to share the latest publication from our lab member, Shiva Nejadebrahim, titled “Multiple O- and an N-glycosylation of the stalk region of the NK cell activation receptor NKp46 mediates its interaction with the Candida glabrata epithelial adhesin 1″.
This study provides, for the first time, a comprehensive glycosylation profile of the NK cell activation receptor NKp46, with a focus on its stalk region. By employing mass spectrometry techniques and recombinant protein production, the research uncovers how both O- and N-glycosylation events contribute to the receptor’s ability to recognise and bind Epa1, a key epithelial adhesin from the fungal pathogen Candida glabrata.
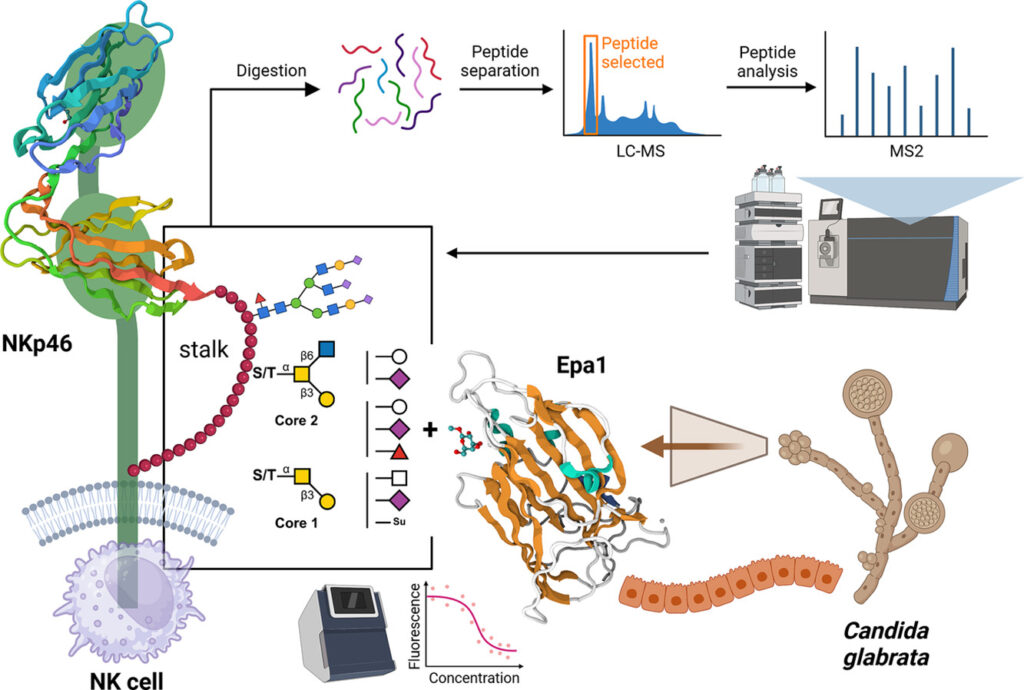
Schematic overview of the interaction between NKp46 on NK cells and Epa1 from Candida glabrata, mediated through complex O- and N-glycosylation patterns on the receptor’s stalk region.
The findings demonstrate that multiple glycosylation sites, including novel O-glycosylation patterns and an essential N-glycan, are critical for NKp46’s interaction with Epa1. These insights highlight a previously underappreciated role of glycosylation in modulating innate immune recognition, with implications for fungal pathogenesis and immune evasion.
Nejadebrahim S., Houserová J., Ječmen T., Kalousková B., Abreu C., Herynek Š., Skořepa O., Bláha J., Vaněk O. (2025): Multiple O- and an N-glycosylation of the stalk region of the NK cell activation receptor NKp46 mediates its interaction with the Candida glabrata epithelial adhesin 1. Int. J. Biol. Macromol. 310, 143037. IF 8.5 DOI: 10.1016/j.ijbiomac.2025.143037