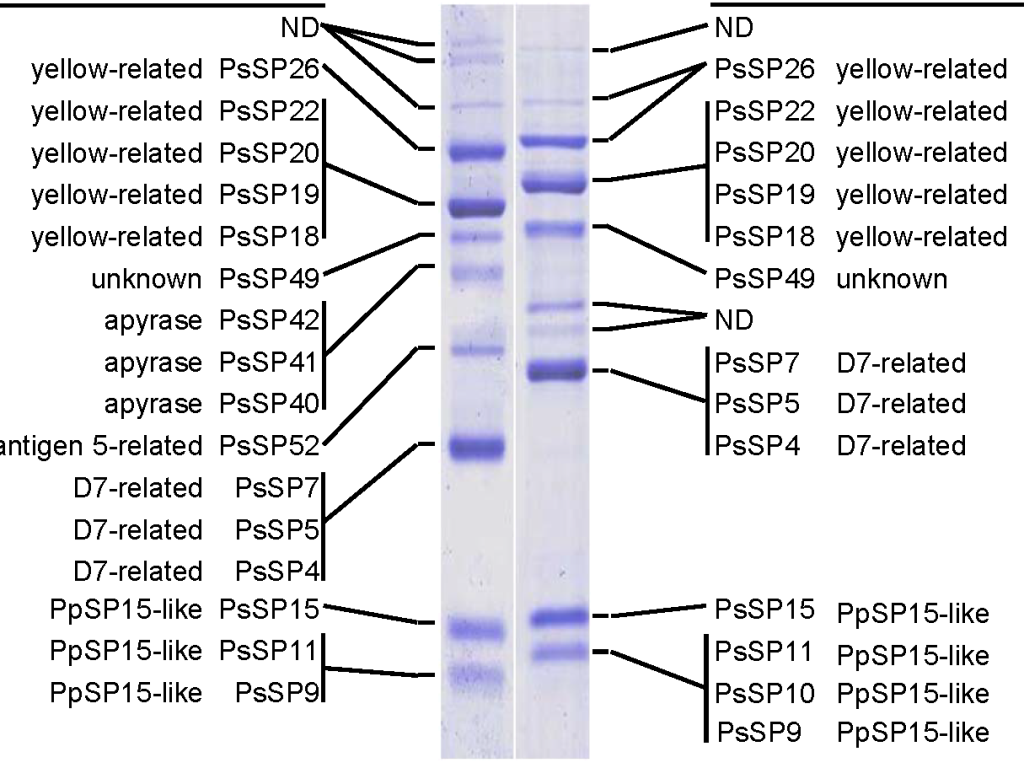
During blood-feeding, sand fly females inject pharmacologically active salivary molecules into the host skin. The salivary compounds are anti-haemostatic and immunomodulatory, helping the female to finish the blood meal. One example of such molecule is hyaluronidase, which degrades extracellular matrix thus allowing the other salivary components to spread within the feeding pool. Another enzyme, apyrase, is responsible for ATP and ADP hydrolysis, thus it inhibits the aggregation of thrombocytes. Yellow-related proteins in sand fly saliva bind biogennic amines such as histamine and serotonine serving as an anticoagulant as well as an antihemostatic molecule.
Another important feature of sand fly saliva is its effect on host immunity. Saliva modifies the site of feeding in favour of transmitted parasites, e.g. Leishmania, so called enhancing effect. Contrary, the immunization (or vaccination) against vector saliva confers the immunity towards the protective immune response against Leishmania infection.
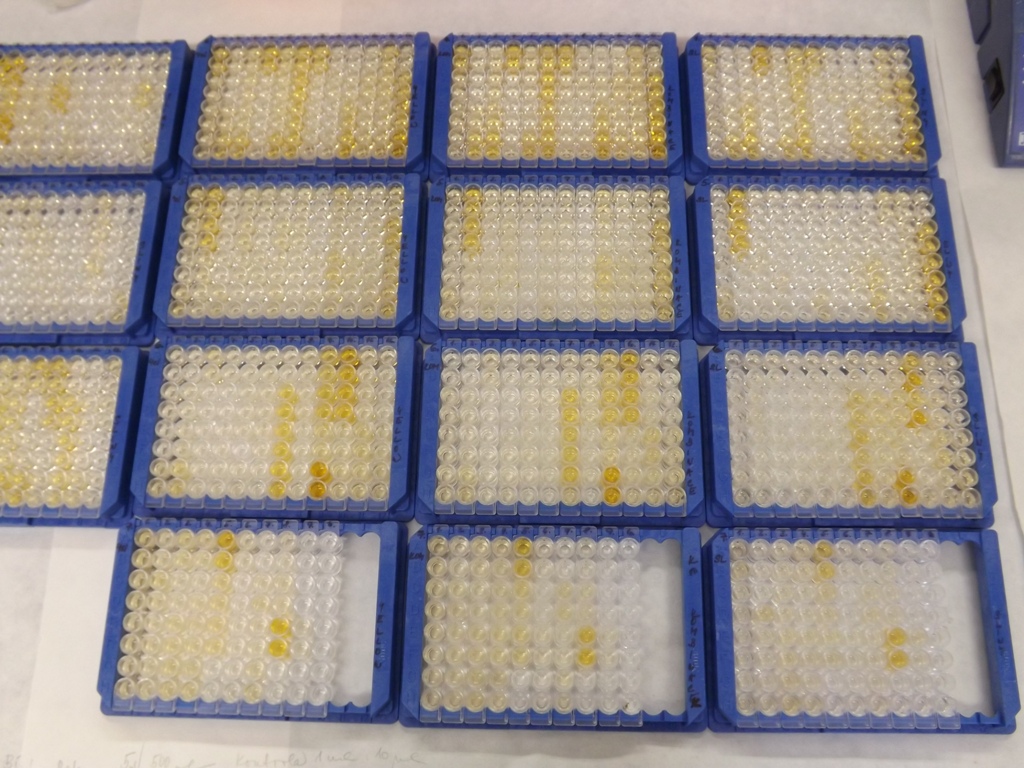
In repeatedly bitten hosts, sand fly saliva elicits humoral as well as cellular immune response. Sand fly saliva injected into the host skin are chemotactic for immune cells, such as monocytes/macrophages, dendritic cells, neutrophils and eosinophils. Antibodies against sand fly saliva are produced in bitten humans as well as in bitten animals, both domestic and wild ones. The anti-saliva antibodies are sand fly species-specific and their level reflects well the time-course and the intensity of sand fly exposure. Thus, the anti-saliva antibodies could be used in endemic areas as a biomarker in vector control programs (repellents, insecticides, mosquito nets) or as a marker of risk of Leishmania infection acquisition.
The utilization of sand fly saliva as an antigen is limited by sand fly colony maintenance and time-consuming dissection of salivary glands. Those limits might be overcome by using salivary recombinant proteins, produced in different expression systems (e.g. E. coli). We have proved this concept in several epidemiological studies that included human and canine sera from areas endemic for cutaneous as well as visceral leishmaniasis. Another step is to develop diagnostic tool to further simplify such test and make it more cost-effective.
- Construction of cDNA libraries of sand fly salivary proteins.
- Characterization of binding properties and enzymatic activities in salivary proteins.
- 3D modeling of salivary proteins.
- Utilization of salivary proteins in a native or a recombinant form to detect anti-saliva antibodies as a marker of sand fly exposure.
- The development of a rapid test to detect anti-saliva antibodies as a marker of sand fly exposure in humans and domestic animals living in areas endemic for leishmaniasis.
- The effect of sand fly saliva on host cellular immunity, in particular on macrophages and on immune response regulation.