Laboratory of bacterial physiology
Group leader: Radovan Fišer
Dept. of Genetics and Microbiology, Charles University
The main aim of our group to correlate data provided by „conventional“ methods
(bacterial physiology, biochemistry and molecular biology) with up-to date biophysical approaches
based mainly on fluorescence spectroscopy and electrophysiology. The topics below illustrate this direction of our
research.
1. Spectrum of Terbium and Calcium Binding Proteins
Terbium exhibits line spectrum (Fig. 1A). Its absorption increases after chelation with some compounds like dipicolinate, or, alternatively, terbium can substitute for calcium in calcium-binding proteins. Hence, for a given protein, its dissociation constant for calcium binding can be obtained measuring phosphorescence intensity dependence on terbium concentration (Fig. 1B).
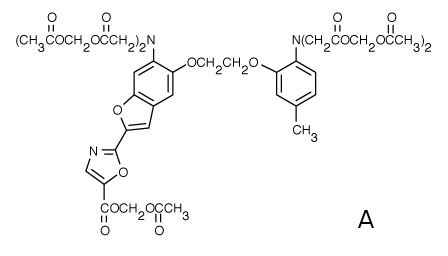
2. FURA and intracellular calcium concentration
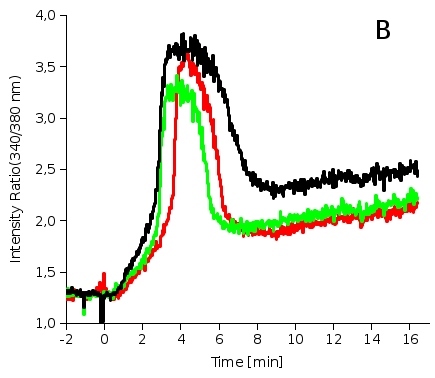
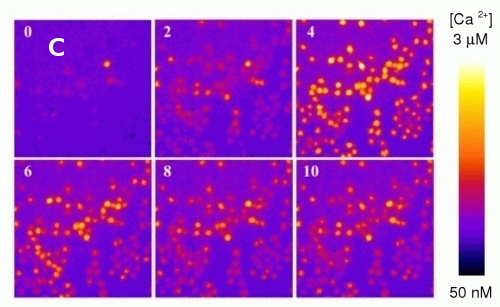
Calcium fluorescence probe FURA (Fig. 2A) is widely used for direct measurements of intracellular calcium concentrations. In order to avoid artifacts resulting from the changes in fluorescence intensity (photobleaching, unstable excitation source, etc.) FURA is used as a ratiometric probe with its intensity ratio (Fluorescence intensity resulting from 340nm excitation/Intensity from 380nm excitation, see Fig. 2B) being sensitive to calcium concentration. Fig. 2B shows increase of intracellular calcium concentration after interaction of mouse macrophages with bacterial toxin. Fig. 2C depicts the same situation using fluorescence microscope. The color scale on the right shows calcium concentration, numbers denote minutes after toxin addition.
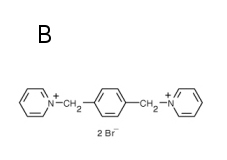
3. Probe ANTS, its quencher DPX and kinetics of the liposome lysis
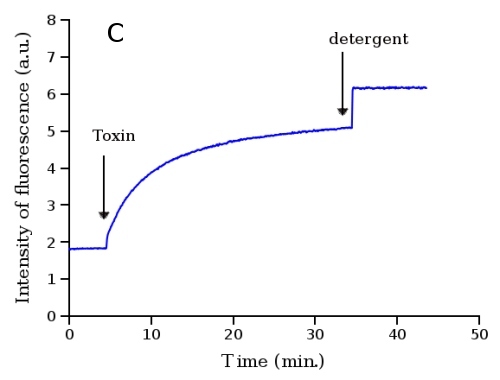
ANTS, naphtalene-derivative fluorescence probe (Fig. 3A), is effectively quenched by DPX (Fig. 3B). The probe with its quencher can be entrapped into liposomes in high concentrations yielding almost no fluorescence due to the quenching. When these liposomes are lysed due to some membrane-disrupting agent (toxin, membrane protein channel, detergent etc.) the fluorescence probe with its quencher are diluted into solution, quenching by DPX decreases and fluorescence of ANTS increases. The time dependence of the fluorescence reflects the kinetics of the liposome leakage (Fig. 3C).
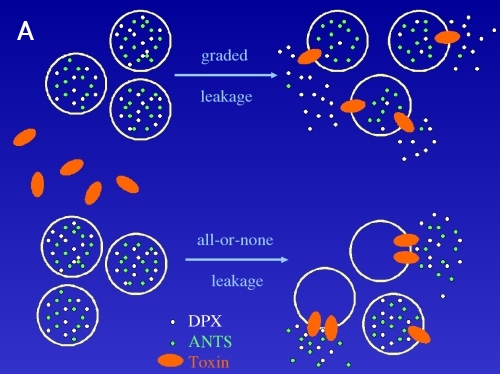
4. Probe ANTS, its quencher DPX and mechanism of liposome lysis
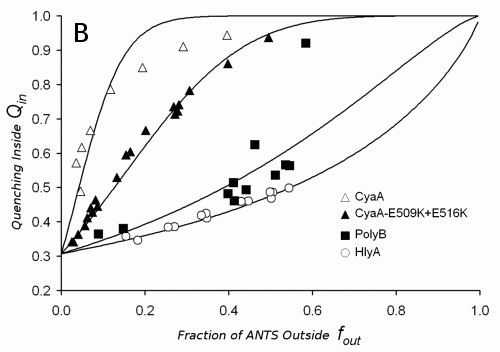
The important details of the liposome leakage caused by an interaction with proteins can also result from using fluorescence probe ANTS and its quencher DPX. One can measure the oligomerization of the protein monomers, mechanism of leakage (graded, all-or-none, see fig. 4A) and selectivity of the toxin channel for cations/anions. From the pattern of the experimental points showed on Fig. 4B these characteristics can be derived or calculated.
5. Laurdan and solid/liquid phases in membranes
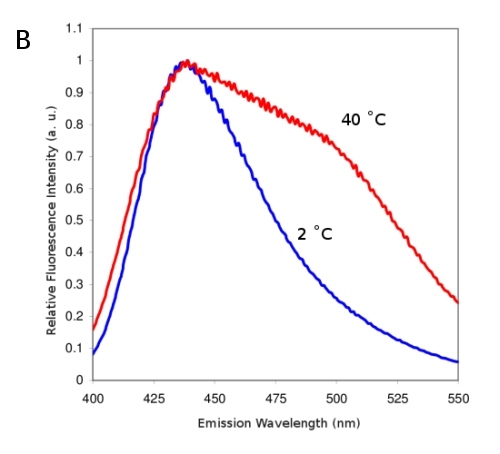
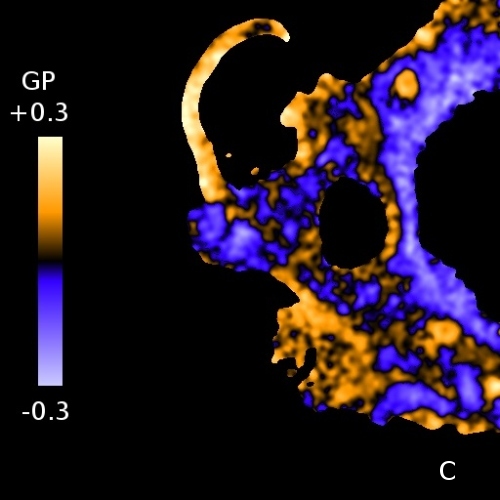
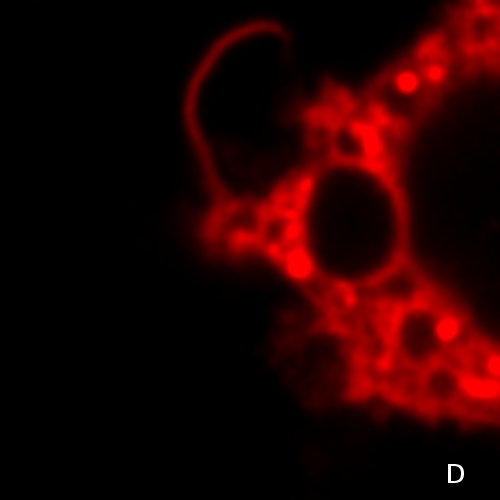
Laurdan fluorescence probe (Fig. 5A) is localized in the membranes. Its emission wavelength is sensitive to the polarity of its environment - more polar environment shifts the emission to higher vawelengths. On Fig. 5B membranes were measured at 2°C and at 40°C. At 40°C the membrane is less ordered and, hence, provides more polar environment for Laurdan. So-called general polarization ratio (GP) of Laurdan fluorescence calculated from intensities at lower and higher emission wavelengths, reflects a proportion of the more viscous (more ordered) phase of the membrane. GP values can be observed employing confocal or fluorescence microscope and the resulting pattern depicts the differences in membrane viscosities (Fig. 5C, color scale on the left denotes GP values). Fig. 5D shows Laurdan intensity for comparison.
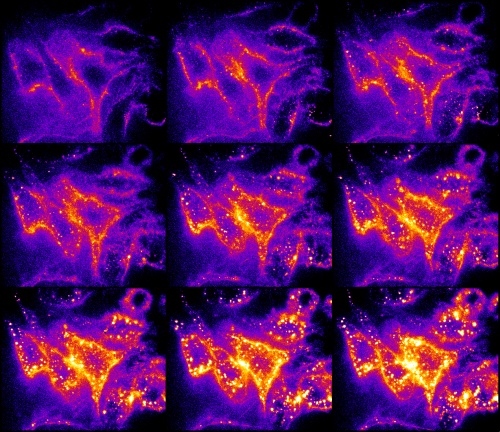
6. Endocytosis of a protein labelled with AlexaFluor 488 in eukaryotic cells
Covalent labelling of the proteins by fluorescence probes can be used for tracking a route of a given protein through an eukaryotic cell. Bacterial toxin was labelled by AlexaFluor 488. Fig. 6 shows the time course of the endocytosis in 2 min. intervals. Mouse macrophages were exposed to labelled toxin. At the beginning, toxin was concentrated at the cytoplasmic membrane and subsequently transported into the cytoplasm via endocytic vesicles.
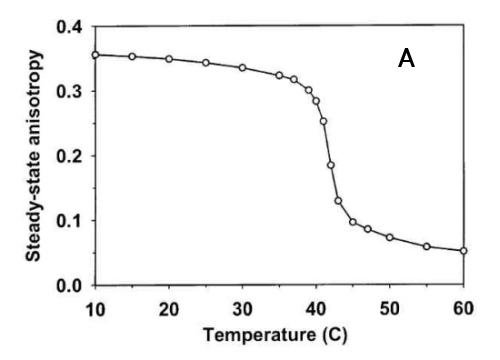
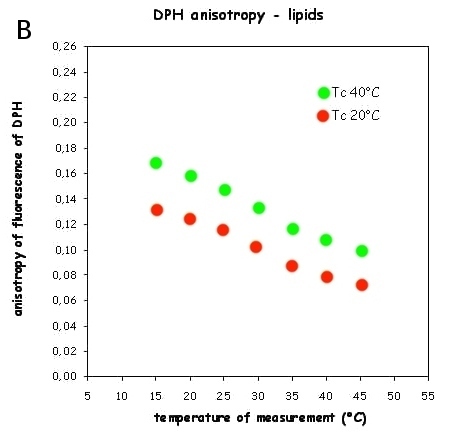
7. DPH, membrane phase transitions and membrane fluidity
Diphenylhexatriene (DPH), a linear hydrophobic membrane fluorescence probe, fits into the membrane hydrophobic core and, to lesser extent, into membrane-water interface. Its rotation in membrane reflects the average membrane "viscosity". After excitation by polarized light, one can calculate fluorescence anisotropy which is very sensitive to the changes of membrane viscosity. Fig. 7A shows the phase transition of synthetic lipid DPPC that has a sharp phase transition about 41.5°C. Bacteria are able to fluidize their membranes at lower cultivation temperature by changing their membrane lipid composition to avoid membrane gelling. Fluorescence anisotropy of bacterial lipids measured in a wide temperature interval allows quantitation of such adaptation for cold (Fig. 7B). Upper curve (Tc40°C, bacteria cultivated at 40°C) shows higher anisotropy (=higher viscosity) while lower one (Tc°20, the same bacteria cultivated at 20°C) shows more fluid lipids due to cold adaptation. If there is no adaptation, both dependences should coincide.
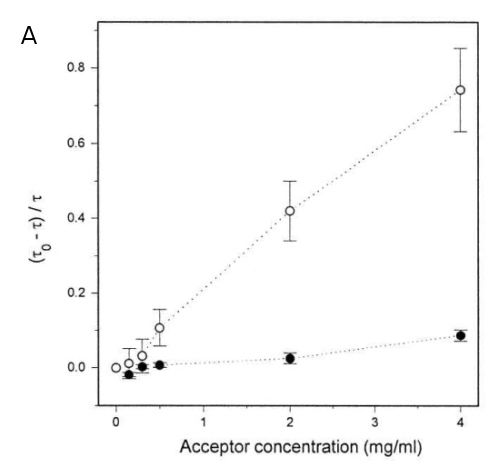
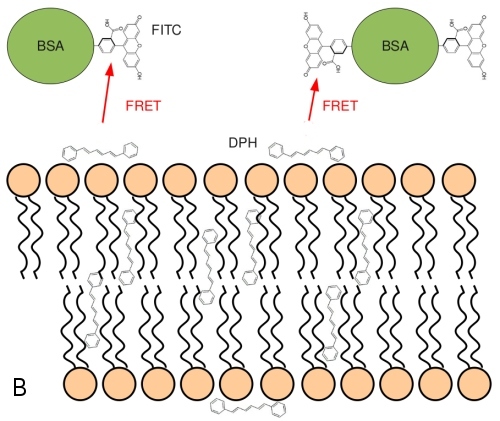
8. DPH - detection of different probe populations by FRET
There are two populations of DPH in the membranes, a major one residing in the membrane hydrophobic core (90%) and a second one (10%) at the membrane -lipid interface. These populations can be detected by FRET, resonance energy transfer between DPH as a donor and FITC-BSA as acceptor molecule. FRET shortens the lifetime Tau of the donor. The inner population is less accessible for the membrane-impermeable FITC-BSA. Fig. 8A shows lower efficiency of FRET between BSA-FITC and inner DPH (lower dependence, black circles) and "outer" DPH (open circles) populations. Tau-zero and tau denote lifetimes in absence and presence of the donor, respectively. Fig. 8B shows the average position of the two DPH populations together with the FITC-labelled BSA.
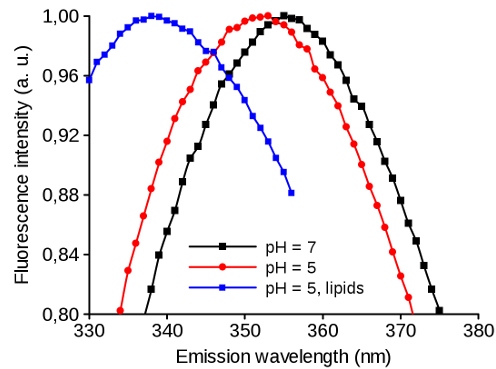
9. Tryptophan as a sensor for membrane-protein interactions
Tryptophan as an "intrinsic fluorescence probe" of the proteins has an emission spectrum that is sensible to its environment. This sensitivity can be used for studying the conformation changes of the protein: a shift of the emission maximum to the left means the more hydrophobic milieu of the tryptophan. Fig. 9 shows viral protein that undergoes conformation change at lower pH making more hydrophobic environment for Trp (Black and Red). After addition of the lipids, the protein at low pH binds into the membrane which shifts the spectrum further to the left and indicates that Trp resides in even more hydrophobic environment (Blue).
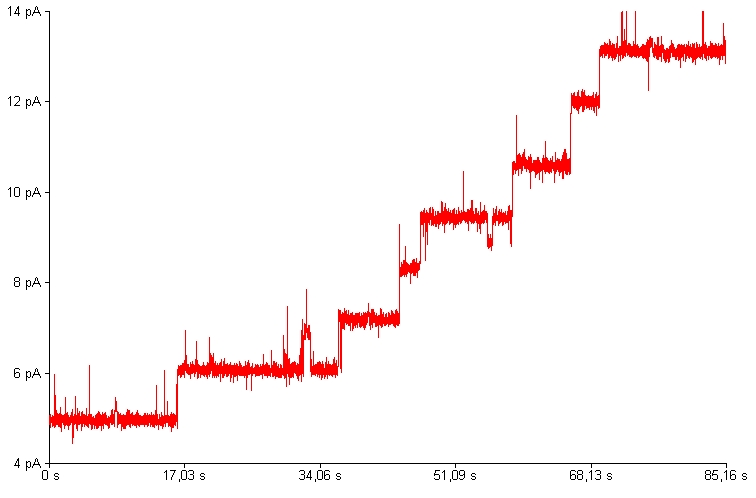
10. Recording of individual membrane channels formed in black lipid membranes
Many bacterial toxins interact with biological membranes and form transmembrane pores. Individual toxin molecules could be characterized by recording of electrical current fluctuations coming from ion passage through the channel. The pore dimensions, dynamics, mechanism of gating or ion selectivity could be deduced from these measurements.